Welcome to our beginner-friendly guide to Planck’s Quantum Theory! In this article, we will explore the fascinating world of quantum physics and how Max Planck’s groundbreaking theory transformed our understanding of the physical universe.
Before Planck’s Quantum Theory, scientists relied on classical physics to explain how things worked. However, they faced significant challenges when explaining certain phenomena, such as the behavior of light and the radiation emitted by hot objects.
Planck’s Quantum Theory revolutionized physics by introducing the concept of quantization. This theory proposes that energy is not continuous, but instead comes in tiny, discrete units called “quanta.” Planck’s constant, a fundamental value in quantum theory, describes the size of these energy packets.
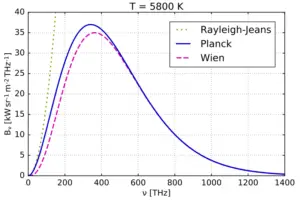
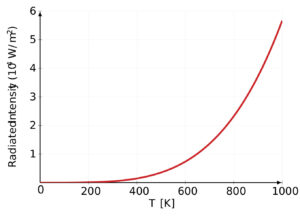
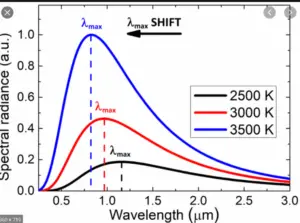
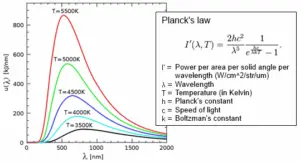
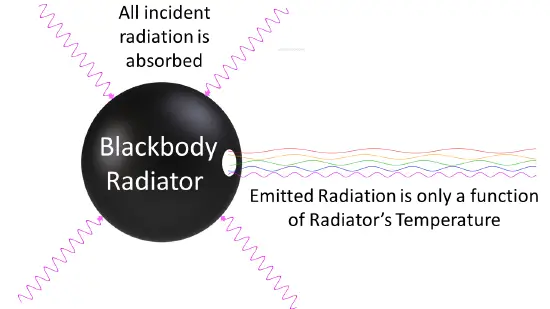
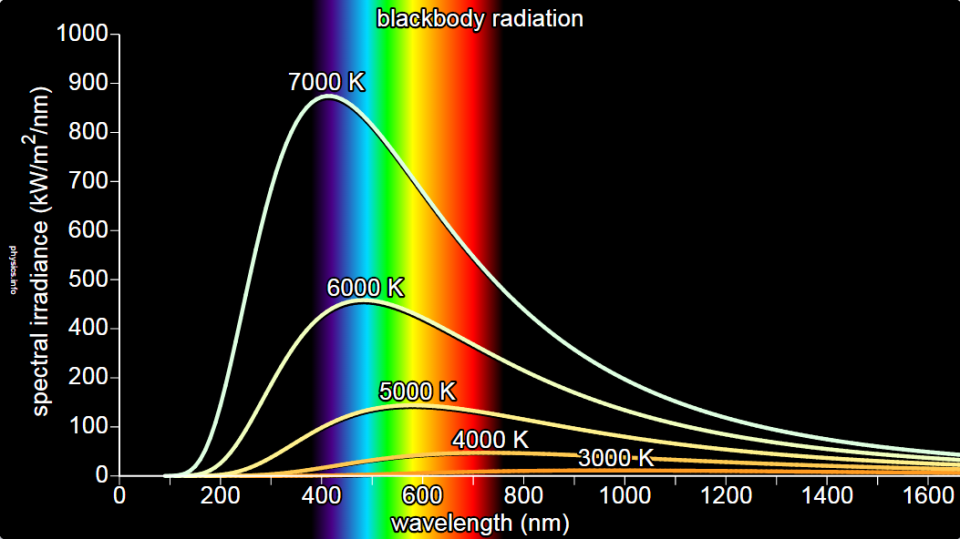
One of the key ideas behind Planck’s Quantum Theory is the understanding of black body radiation, which refers to how objects emit and absorb electromagnetic radiation at different temperatures. Planck’s theory successfully explained the observed behavior of black bodies and resolved a long-standing problem known as the ultraviolet catastrophe.
Additionally, Planck’s Quantum Theory introduced the notion of particles of light called photons. These particles possess both wave-like and particle-like properties, a concept known as wave-particle duality. This idea challenged the classical notion that light behaves solely as a wave or a particle and laid the foundation for the development of quantum mechanics.
Throughout this article, we will explore these concepts in a beginner-friendly manner, exploring their historical context and practical implications. Let’s embark on this journey to uncover the mysteries of Planck’s Quantum Theory together!
Read Also:
- Rutherford atomic model: postulates, observations, and limitations, class 11
- Thomson model of atom: postulates, drawbacks, & significance, class 11
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Discovery of Electron class 11: chemistry, NCERT
- Discovery of proton class 11: chemistry NCERT
- Exploring the Bohr Atomic Model: A Comprehensive Guide
- Wave nature of electromagnetic radiation, class 11
- Particle nature of electromagnetic radiation, class 11
Understanding the historical context
To fully appreciate the significance of Planck’s Quantum Theory, it’s essential to understand the historical context in which it emerged. Before Planck’s groundbreaking work, scientists were heavily influenced by classical physics theories, which had successfully explained many phenomena but struggled to account for certain observations.
In the late 19th century, scientists faced challenges in explaining the behavior of light and the radiation emitted by hot objects, known as black body radiation. According to classical physics, these phenomena should have been explained by continuous waves. However, experimental data revealed discrepancies that could not be reconciled with existing theories.
Max Planck, a German physicist, entered the scene and made a remarkable breakthrough. In 1900, while studying black body radiation, he proposed a revolutionary idea: energy is not continuous but rather comes in discrete packets, or “quanta.” This concept flew in the face of traditional physics, which assumed that energy could take any value and be divided infinitely.
Planck’s Quantum Theory introduced the concept of quantization, which states that energy can only exist in specific, discrete amounts. He derived a mathematical formula that accurately described the distribution of energy at different wavelengths for black bodies, resolving the long-standing problem known as the ultraviolet catastrophe.
At the time, Planck himself did not fully grasp the profound implications of his theory. He initially proposed it as a mathematical trick to fit the experimental data, rather than a fundamental change in our understanding of physics. However, his work laid the foundation for a paradigm shift in scientific thinking.
Planck’s Quantum Theory challenged the prevailing beliefs and paved the way for a deeper exploration of the microscopic world. It led to the development of quantum mechanics, a branch of physics that describes the behavior of particles at the atomic and subatomic levels.
Key concepts and principles underlying Planck’s quantum theory
Now let’s discuss the key concepts and principles underlying Planck’s Quantum Theory. These fundamental ideas revolutionized our understanding of the physical world and laid the groundwork for the development of quantum mechanics.
- Quantization
Planck’s Quantum Theory introduced the concept of quantization, which states that energy is not continuous but exists in discrete units called “quanta.” This means energy can only exist in specific, quantized amounts rather than being infinitely divisible. - Planck’s Constant
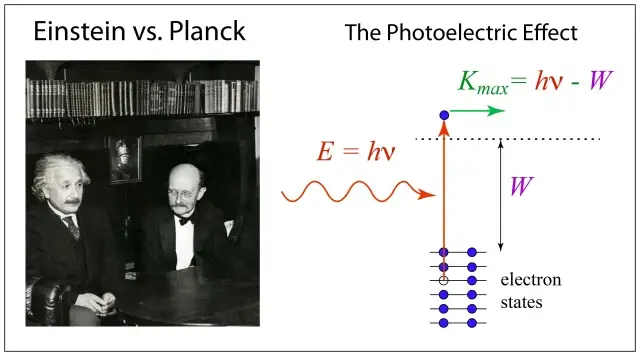
Planck’s constant, denoted by the symbol ‘h,’ is a fundamental value in quantum theory. It relates the energy of a quantum (E) to its frequency (ν) through the equation E = hν. Planck’s constant determines the size of energy packets, or quanta. - Black Body Radiation
Planck’s Quantum Theory provided a breakthrough explanation for black body radiation. A black body is an idealized object that absorbs and emits all frequencies of electromagnetic radiation. Planck’s theory successfully described how radiation energy is distributed across different wavelengths for a given temperature, resolving the problematic predictions of classical physics known as the ultraviolet catastrophe. - Planck-Einstein Relation
Planck’s Quantum Theory laid the foundation for Albert Einstein’s work on the photoelectric effect. Einstein proposed that light consists of particles called photons, each carrying a discrete amount of energy. The energy of a photon is directly proportional to its frequency, as given by the Planck-Einstein relation: E = hν. This relationship established the wave-particle duality of light, suggesting that light exhibits both wave-like and particle-like behavior. - Wave-Particle Duality
The wave-particle duality is a fundamental concept in quantum theory. It suggests that particles like photons and electrons can exhibit wave-like and particle-like properties depending on the experimental setup. This duality challenges the classical notion that particles and waves are distinct entities and highlights the inherent probabilistic nature of quantum phenomena. - Uncertainty Principle
The uncertainty principle, formulated by Werner Heisenberg, states that there is a fundamental limit to how precisely certain pairs of physical properties, such as position and momentum, can be simultaneously known. This principle highlights the inherent limitations in our ability to measure and predict the behavior of quantum systems.
Read Also
- AC circuit containing an inductor only
- AC circuit containing resistor only, class 12
- Representation of AC current and voltage by phasor diagram
- AC circuit containing resistor and inductor in series
Implications of Planck’s quantum theory and its applications
Planck’s Quantum Theory has had far-reaching applications and profound implications across various fields of science and technology. Here are some of the key areas where the theory has had a significant impact:

- Quantum Mechanics
Planck’s Quantum Theory laid the groundwork for the development of quantum mechanics, a branch of physics that describes the behavior of particles at the atomic and subatomic levels. Quantum mechanics has provided a more accurate and comprehensive understanding of the microscopic world, enabling scientists to explain phenomena such as atomic structure, particle interactions, and the behavior of matter and energy on the quantum scale. - Electronics and Computing
The principles of quantum mechanics derived from Planck’s theory have revolutionized the fields of electronics and computing. Quantum computers, based on the principles of superposition and entanglement, have the potential to solve complex problems at an exponentially faster rate than classical computers. Additionally, quantum mechanics has led to the development of technologies such as quantum cryptography and quantum sensors, which offer enhanced security and sensing capabilities. - Optics and Lasers
The understanding of wave-particle duality provided by Planck’s Quantum Theory has significantly advanced the field of optics. Lasers, for instance, rely on the principles of quantum mechanics to generate coherent and intense light beams. Applications of lasers range from telecommunications to medical treatments, precision manufacturing, and scientific research. - Solid-State Physics
Planck’s Quantum Theory has greatly influenced the field of solid-state physics, which studies the properties of solids and their behavior at the atomic and electronic levels. The theory provides insights into phenomena such as electrical conductivity, magnetism, and the behavior of semiconductors, leading to the development of numerous electronic devices, including transistors, diodes, and integrated circuits. - Energy Technologies
Quantum theory has played a crucial role in the advancement of energy technologies. For example, the understanding of energy quantization and electron behavior has contributed to the development of efficient solar cells, which convert sunlight into electricity. Quantum mechanics has also facilitated advancements in energy storage, catalysis, and materials science, leading to more sustainable and efficient energy solutions. - Fundamental Understanding of the Universe
Planck’s Quantum Theory has fundamentally reshaped our understanding of the universe at its most fundamental level. It has challenged our classical intuitions and provided a framework that accurately describes the behavior of matter and energy on the quantum scale. The theory has deepened our knowledge of the building blocks of the universe, helping us unravel the mysteries of subatomic particles, quantum fields, and the origins of the cosmos.
The implications of Planck’s Quantum Theory extend far beyond the examples mentioned here, impacting all aspects of modern science and technology. Its influence continues to drive new discoveries and a deeper understanding of our universe’s quantum nature.
Planck’s quantum theory and the Wave-Particle Duality
Now let’s explore the intriguing concept of wave-particle duality, which lies at the center of Planck’s Quantum Theory. This concept challenges our classical understanding of particles and waves, revealing the dual nature of fundamental entities like light and matter.
- Understanding Wave-Particle Duality
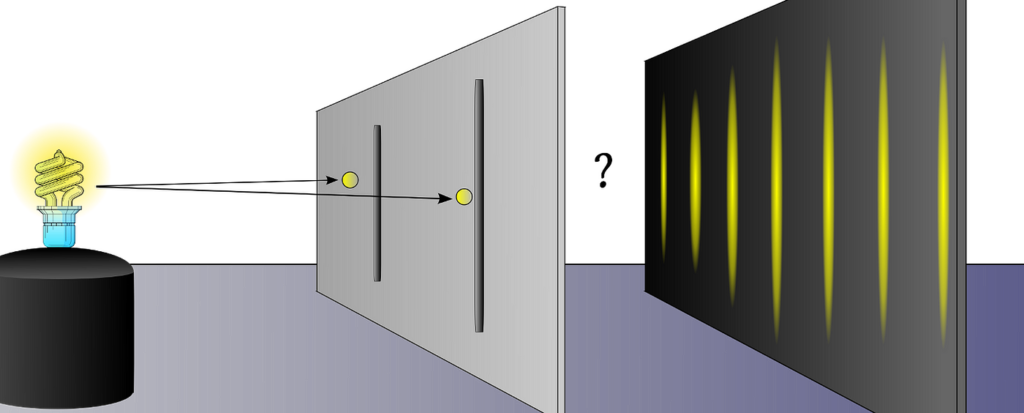
Wave-particle duality refers to the idea that particles, such as photons or electrons, can exhibit both wave-like and particle-like properties depending on the experimental context. This duality suggests that these entities have characteristics of both waves and particles, blurring the boundaries between the two. - The Double-Slit Experiment
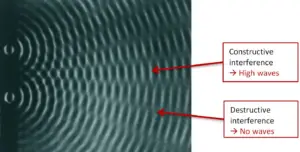
One of the most famous experiments demonstrating wave-particle duality is the double-slit experiment. In this experiment, a beam of particles or waves is directed at a barrier with two narrow slits. Surprisingly, when particles such as electrons or photons are sent through the slits one at a time, an interference pattern similar to that of waves is observed on a screen behind the barrier. This interference pattern indicates that particles can interfere with themselves, just like waves do. - Particle-Like Behavior
Despite exhibiting wave-like properties, particles also demonstrate particle-like behavior. For example, when individual particles are detected or measured, they behave as discrete entities, showing localized positions and distinct properties. This behavior is reminiscent of classical particles, leading to the understanding that particles can exist as quantized entities with specific properties. - The Uncertainty Principle
The wave-particle duality is intimately connected to the uncertainty principle, a fundamental principle in quantum mechanics. The uncertainty principle, formulated by Werner Heisenberg, states that there is a fundamental limit to the precision with which certain pairs of physical properties, such as position and momentum, can be simultaneously known. This principle highlights the inherent uncertainty and probabilistic nature of quantum systems. - Applications and Significance
The concept of wave-particle duality has profound implications across various scientific disciplines. In the field of optics, it helps explain phenomena like diffraction and interference, enabling the development of technologies such as lasers and holography. In particle physics, it underlies the behavior of subatomic particles and their interactions. Additionally, wave-particle duality is instrumental in understanding phenomena such as electron microscopy, quantum tunneling, and the behavior of matter in quantum systems.
Wave-particle duality challenges classical intuitions and highlights the need for a new paradigm in quantum physics. It reveals the intricate nature of particles and waves, inviting further exploration.
Read Also:
- Maxwell’s equations class 12: integral form, differential form, applications, and explanation
- Displacement current class 12: definition, modification, formula, and properties
- Electromagnetic waves class 12: definition, equation, graphical representation, and applications
- Electromagnetic spectrum class 12
Criticisms and Controversies
While Planck’s Quantum Theory has revolutionized our understanding of the physical world, it has also faced criticism and sparked controversies among scientists and philosophers. In this section, we will explore some of the main criticisms and controversies surrounding quantum theory.
- Interpretations of Quantum Mechanics
One major source of controversy is the interpretation of quantum mechanics. Different interpretations, such as the Copenhagen interpretation, the many-worlds interpretation, and the pilot-wave theory, offer distinct explanations for the behavior of quantum systems. These interpretations often lead to philosophical debates about the nature of reality, determinism, and the role of the observer in quantum phenomena. - Measurement Problem
The measurement problem is a key challenge in quantum mechanics. It arises from the fact that the act of measurement appears to collapse the wave function, determining the outcome of an observation. This collapse of the wave function is not fully understood, and various interpretations offer different explanations. Some critics argue that the measurement problem reveals a fundamental flaw or incompleteness in our current understanding of quantum mechanics. - Determinism vs. Indeterminism
Quantum mechanics introduces inherent randomness and probabilistic outcomes into the behavior of particles and waves. This departure from classical determinism, where the future state of a system is entirely predictable from its initial conditions, has been met with criticism. Some physicists and philosophers argue that the indeterministic nature of quantum theory challenges our intuitions and raises questions about the fundamental nature of reality. - Compatibility with General Relativity
Another point of contention lies in reconciling quantum mechanics with Albert Einstein’s theory of general relativity, which describes gravity and the behavior of spacetime on a large scale. The lack of a consistent framework that unifies quantum mechanics and general relativity, known as the theory of quantum gravity, is a subject of ongoing research and debate. - Epistemological and Ontological Questions
Quantum theory raises profound epistemological and ontological questions about the nature of knowledge and reality. The probabilistic nature of quantum phenomena and the role of observers in collapsing the wave function have led to discussions on the nature of consciousness, the observer’s influence, and the limits of scientific knowledge. - Experimental Challenges
Quantum mechanics has been confirmed by numerous experiments and has impressive predictive power. However, some critics argue that certain interpretations of quantum theory, such as the many-worlds interpretation, are difficult to test experimentally, making them more speculative in nature.
It is important to note that while these criticisms and controversies exist, quantum mechanics remains one of the most successful and empirically verified theories in science. The ongoing debates and inquiries surrounding the theory reflect the ongoing quest for a deeper understanding of the quantum nature of our universe.
Read Also
- Uncertainty in measurement chemistry, class 11 NCERT
- Laws of chemical combination: chemistry class 11, NCERT
- John dalton’s atomic theory: postulates and limitations, class 11, NCERT
Conclusion
Planck’s Quantum Theory has had a profound impact on our understanding of the physical world, revolutionizing physics and shaping numerous scientific and technological advancements. By introducing the concept of quantization and the notion of energy quanta, Planck laid the foundation for the development of quantum mechanics.
Key concepts and principles such as quantization, Planck’s constant, black body radiation, the Planck-Einstein relation, wave-particle duality, and the uncertainty principle have reshaped our understanding of particles, waves, and the fundamental nature of reality. These concepts have found applications in areas such as quantum mechanics, electronics, optics, solid-state physics, energy technologies, and our fundamental understanding of the universe.
While quantum theory has faced criticisms and controversies, including debates over interpretations, the measurement problem, determinism, compatibility with general relativity, and epistemological questions, it remains one of the most successful and empirically tested theories in science. The ongoing discussions and inquiries surrounding the theory reflect the ongoing pursuit of knowledge and the quest for a deeper understanding of the quantum realm.
Planck’s Quantum Theory has not only expanded our understanding of the microscopic world but has also paved the way for groundbreaking technologies and practical applications. From quantum computing to precision optics and energy technologies, the principles of quantum mechanics derived from Planck’s theory continue to drive innovation and push the boundaries of scientific exploration.
As we continue to unravel the mysteries of the quantum world, Planck’s Quantum Theory stands as a cornerstone of modern physics, inspiring scientists, researchers, and thinkers to delve deeper into the fundamental nature of our universe and unlock new frontiers of knowledge and discovery.
Stay tuned with Laws Of Nature for more useful and interesting content.