Discovery of Electron: Introduction
Long before scientists had any clue about the existence of sub-atomic particles, John Dalton came up with an idea that revolutionized the way we think about the matter. In 1808, he proposed the Atomic Theory, which stated that matter is made up of tiny, indivisible particles called atoms that are indestructible.
Dalton’s theory also pointed out that atoms of the same element have identical properties, but they differ from atoms of other elements. Atoms can neither be created nor destroyed. Furthermore, atoms combine in a fixed, whole-number ratio to form molecules. These groundbreaking postulates formed the foundation of modern atomic theory.

However, as with all scientific theories, Dalton’s postulates were subject to scrutiny and experimentation. Enter Faraday, who discovered that charged particles were responsible for the flow of electricity. This finding paved the way for the discovery of sub-atomic particles.
One scientist who capitalized on this discovery was William Crookes. He used a cathode ray tube to study the behavior of electrons, leading to important breakthroughs in studying atomic structure. Crookes’ experiments showed that the cathode ray was composed of negatively charged particles that he dubbed “cathode rays.”
But Sir J.J. Thomson took this groundbreaking discovery to the next level. He proved the existence of electrons as negatively charged sub-atomic particles within atoms, which fundamentally altered our understanding of matter. Thomson’s work ultimately led to the discovery of other sub-atomic particles such as protons and neutrons, and it paved the way for our modern understanding of the structure of atoms.
So, while Dalton’s Atomic Theory may have been proven incorrect in some respects, it was the foundation on which modern atomic theory was built. And thanks to scientists like Faraday, Crookes, and Thomson, we now have a much better understanding of the fascinating and complex world of sub-atomic particles.
In this article, we will discuss the discovery of electrons using the Cathode ray tube by William Crooke and later the actual discovery of electrons by J.J. Thomson.
What are Cathode Ray and Cathode ray tubes?
What are Cathode Rays?
A cathode ray is a stream of electrons that are emitted from the negatively charged electrode (cathode) in a vacuum tube. The electrons are accelerated by an electric field and travel in a straight line until they are deflected by magnetic or electric fields. Cathode rays have a negative charge and are used in a wide range of applications, including television and computer displays, X-ray machines, and particle accelerators.
What are Cathode ray tubes?
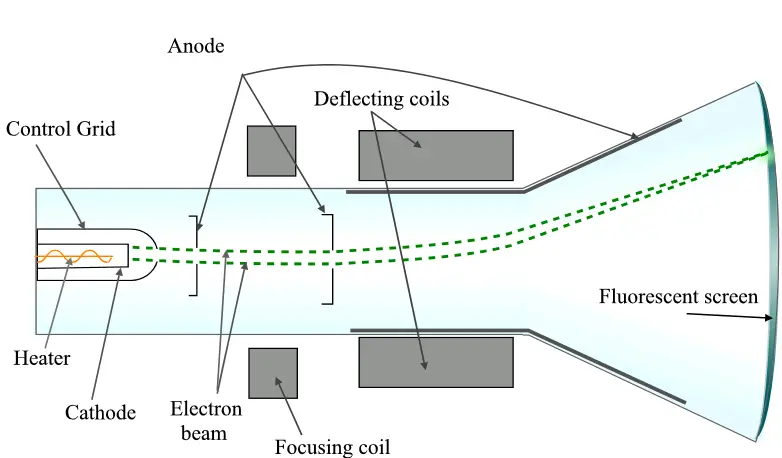
A cathode ray tube (CRT) is a vacuum tube that uses cathode rays to produce images on a fluorescent screen. The CRT was first invented in the late 19th century and was widely used in early televisions and computer monitors. The basic design of a CRT consists of an electron gun that produces a beam of cathode rays, which are then focused and directed onto a fluorescent screen. The screen emits light when struck by the electrons, producing an image that can be viewed by the user.
Cathode ray tubes have largely been replaced by newer display technologies such as LCDs and OLEDs. However, they remain important in certain applications such as oscilloscopes and other laboratory equipment.
William Crooke’s discovery of the electron
William Crookes was a British scientist who made significant contributions to the study of electricity and magnetism in the late 19th century. One of his most notable discoveries was the identification of the electron, which he achieved through his work with cathode rays.
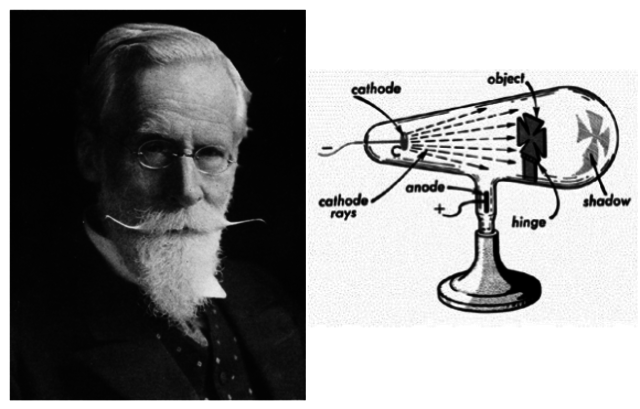
In the late 19th century, scientists were studying the behavior of electricity in gas-filled tubes known as cathode ray tubes. Crookes developed a type of cathode ray tube that allowed him to study the properties of the cathode rays in more detail. He observed that when an electric current was passed through the tube, a stream of negatively charged particles was emitted from the cathode and traveled toward the anode.
Crookes conducted a series of experiments to study the properties of these cathode rays. He observed that they could be deflected by electric and magnetic fields, which suggested that they had a charge. He also found that they could produce a bright spot of light when they struck a fluorescent screen, which suggested that they had energy.
Crookes concluded that the cathode rays were a stream of negatively charged particles, which he called “radiant matter“. He believed that these particles were a fundamental component of matter and that they could exist independently of atoms.
Crookes’ work was groundbreaking because it provided the first evidence for the existence of subatomic particles. His discovery paved the way for further research into the properties of electrons and their role in atomic structure, which led to the development of modern physics and electronics.
Read Also
- Atomic mass and molecular mass: chemistry class 11, NCERT
- John dalton’s atomic theory: postulates & limitations, class 11, NCERT
- Laws of chemical combination: chemistry class 11, NCERT
- Matter | Nature of matter | classification of matter, class 11 | some basic concepts of chemistry
- Stoichiometry and stoichiometric calculations class 11, NCERT
J.J. Thomson and The Discovery of Electron
Sir J.J. Thomson’s discovery of the electron was a groundbreaking achievement that transformed the field of physics and chemistry. In the late 1800s, scientists were trying to understand the nature of electricity and how it behaves in different materials. This quest led to the development of cathode ray tubes, which were instrumental in Thomson’s discovery of the electron.

Thomson began his work by creating a vacuum inside a cathode ray tube (Cathode ray tubes are vacuum-sealed glass tubes that have had most of the air removed.). He then applied a high voltage to the tube, which caused a stream of particles to flow from the negatively charged cathode to the positively charged anode. This stream of particles is now known as a cathode ray.
Thomson noticed that when he placed a magnet near the tube, the path of the cathode rays was deflected. He concluded that the cathode rays were negatively charged, as they were attracted to the positively charged plate and repelled by the negatively charged plate.
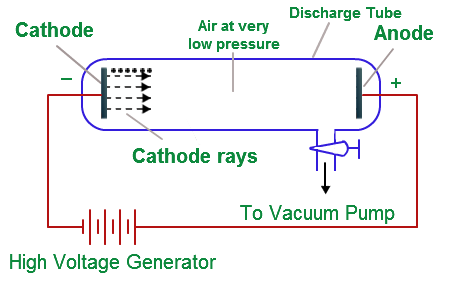
To determine the properties of these particles, Thomson performed a series of experiments on the cathode rays. One of his key experiments involved measuring the ratio of the charge to the mass of the cathode rays. Thomson used a magnetic field to deflect the cathode rays and a parallel electric field to control their motion. By varying the strength of the fields, Thomson was able to calculate the charge-to-mass ratio of the particles.
Thomson discovered that the cathode rays had a much smaller mass than any previously known subatomic particle and that they carried a negative charge. He proposed that these particles were a new subatomic particle, which he called “corpuscles.” This later became known as the electron.
Thomson’s discovery of the electron had profound implications for our understanding of atomic structure. Prior to his work, atoms were thought to be indivisible and indestructible. However, Thomson’s discovery of the electron showed that atoms were actually composed of smaller subatomic particles. This discovery paved the way for the development of the modern atomic model, which includes electrons orbiting around a central nucleus composed of protons and neutrons.
Summary: J.J. Thomson discovered the electron by studying the behavior of cathode rays in a vacuum tube. His experiments led him to conclude that these rays were composed of negatively charged subatomic particles with a much smaller mass than any previously known subatomic particle. His discovery transformed our understanding of the atomic structure and paved the way for future discoveries in the field of subatomic particles
Thomson’s discovery of electron: Key findings
J.J. Thomson’s work on the discovery of the electron and its properties was groundbreaking and had many important findings. Here are some of the key findings from Thomson’s work:
- Discovery of the electron: Thomson discovered the electron as a subatomic particle that carries a negative electric charge. He proposed that these particles were present in all matter and were fundamental building blocks of atoms.
- Cathode rays: Thomson studied the behavior of cathode rays, which are streams of electrons that are emitted from the negatively charged cathode in a vacuum tube. He showed that these rays were negatively charged and that they could be deflected by electric and magnetic fields.
- The charge-to-mass ratio of the electron: Thomson measured the charge-to-mass ratio of the electron by studying the deflection of cathode rays in a magnetic field. He found that the ratio was much larger than that of any previously known subatomic particle, suggesting that the electron had a much smaller mass than any other particle.
- Plum pudding model of the atom: Based on his work on the charge-to-mass ratio of the electron, Thomson proposed a model of the atom in which electrons were embedded in a positively charged sphere. This model became known as the plum pudding model of the atom.
- Isotopes: Thomson’s work on the properties of the electron led to the discovery of isotopes, which are atoms of the same element that have different numbers of neutrons. This was an important discovery that had implications for the fields of chemistry and physics.
Summary: Thomson’s work on the discovery of the electron and its properties had a profound impact on our understanding of the atomic structure and the behavior of matter at the subatomic level. His findings laid the groundwork for future discoveries in the field of subatomic particles and helped to shape our modern understanding of the nature of matter.
Read Also:
- Percentage composition chemistry class 11: formula, definition, examples, NCERT
- Mole concept class 11 | definition, formula & solved examples, NCERT
- Uncertainty in measurement chemistry, class 11 NCERT
- Empirical formula & molecular formula: chemistry class 11
What is an Electron, and its properties?
An electron is a subatomic particle that carries a negative electric charge. It is discovered by J.J. Thomson. It is one of the fundamental building blocks of matter and is found in all atoms, along with protons and neutrons. Electrons are extremely small, with a mass that is approximate 1/1836th that of a proton.
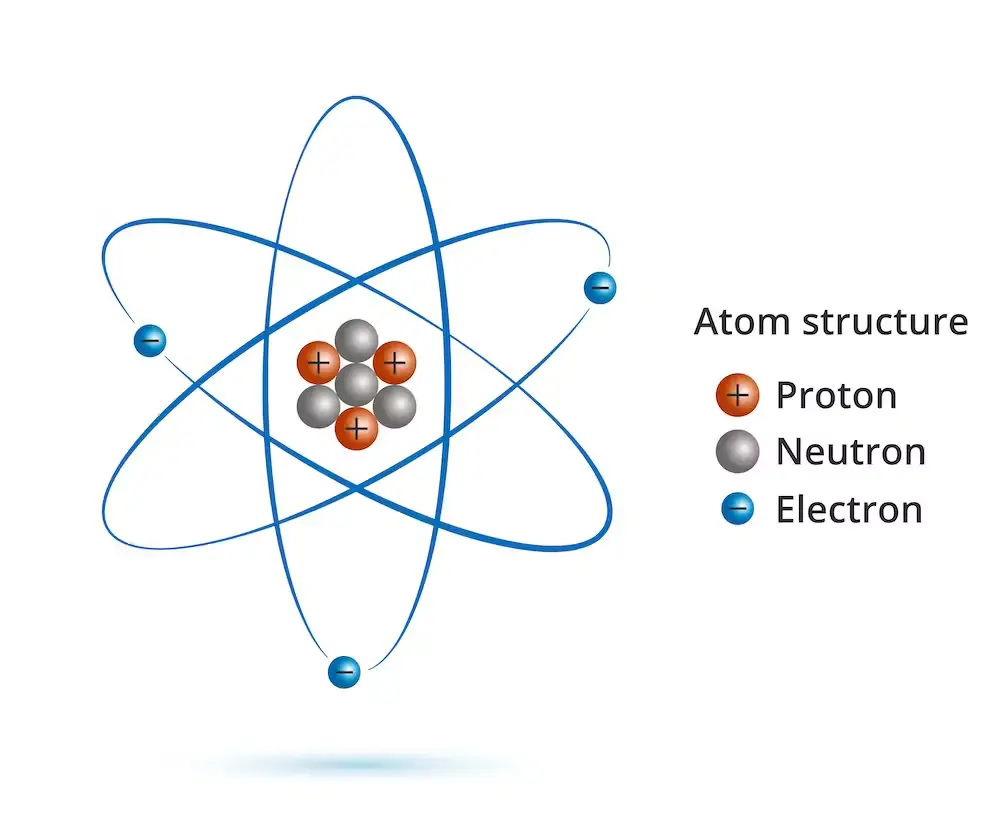
Here are some of the key properties of electrons:
- Charge: Electrons carry a negative electric charge, which is equal in magnitude to the positive charge of a proton. The charge of an electron is -1.602 x 10^-19 coulombs.
- Mass: Electrons have a mass of approximately 9.11 x 10^-31 kilograms.
- Spin: Electrons have a property called spin, which is a quantum mechanical property that describes their intrinsic angular momentum. Electrons can have either “spin up” or “spin down” orientations.
- Energy levels: Electrons in an atom occupy energy levels, or shells, that are defined by their distance from the nucleus. Electrons in higher energy levels have more energy and are farther away from the nucleus.
- Interactions: Electrons interact with each other and with other particles through electromagnetic force, which is one of the four fundamental forces of nature. Electrons can also interact with other particles through the weak force, which is responsible for nuclear decay.
- Wave-particle duality: Electrons have both wave-like and particle-like properties, which is known as wave-particle duality. This means that electrons can behave as both waves and particles, depending on the experiment or observation.
What was the significance of the discovery of electrons?
The discovery of electrons was a significant milestone in the history of science and had a profound impact on our understanding of the physical world. Here are some of the key significance of the discovery of electrons:
- The existence of subatomic particles: The discovery of electrons provided the first evidence that atoms were not indivisible and that they contained smaller particles. This paved the way for further discoveries of subatomic particles such as protons, neutrons, and quarks.
- Development of atomic theory: The discovery of electrons led to the development of a new model of atomic structure, which helped to explain the chemical and physical properties of elements. This model, known as the Bohr model, proposed that electrons orbit the nucleus in discrete energy levels.
- Advances in technology: The discovery of electrons led to the development of electronics, which has revolutionized our world. It enabled the creation of new technologies such as televisions, computers, and smartphones, and led to the development of modern communication systems.
- New fields of study: The discovery of electrons opened up new fields of study such as particle physics, which seeks to understand the behavior of subatomic particles. It also led to the development of new research tools such as particle accelerators, which allow scientists to study particles at high energies.
Frequently Asked Questions – FAQs
Who is the father of the electron?
J.J. Thomson is often referred to as the “father of the electron” because of his pioneering work in the discovery and study of electrons.
What is the charge of one electron?
The charge of one electron is -1.602 x 10^-19 coulombs. The charge of an electron is negative and opposite in sign to the charge of a proton, which is +1.602 x 10^-19 coulombs.
Electrons are the negatively charged subatomic particles that orbit the nucleus of an atom and are responsible for chemical reactions and electrical conductivity.
who is credited with the discovery of electrons?
The discovery of electrons is credited to J.J. Thomson, a British physicist who conducted a series of experiments in 1897 on cathode rays using a cathode ray tube.
Through these experiments, he observed that the cathode rays could be deflected by electric and magnetic fields, suggesting that they had a charge. By measuring the extent of deflection and the strength of the electric and magnetic fields, he was able to calculate the charge-to-mass ratio of the particles in the cathode rays.
Thomson concluded that these particles were negatively charged subatomic particles, which he called electrons. His discovery revolutionized the field of atomic theory and led to the development of new technologies such as electronics.
Who discovered cathode rays?
Cathode rays were discovered by Sir William Crookes, a British physicist, and chemist, in 1879.
What is the significance of electrons?
Electrons are of immense significance in modern physics and technology. They are the negatively charged subatomic particles that are responsible for chemical reactions and electrical conductivity in the matter.
The discovery of electrons has led to the development of electronics, which is the basis of modern technology. The study of electrons has also revolutionized our understanding of the atomic structure and quantum mechanics.
Stay tuned with Laws Of Nature for more useful and interesting content.