Whenever we open our eyes, we see lots of matters around us. In fact, we are surrounded by various different types of matters. In this world, nothing is constant, everything is changing with time. The matters around us are also changing with time chemically or physically. Due to their chemical or physical change, they are constantly changing from one matter to another.
Chemistry is the study of the transformation of matter from one form to the other. These transformations usually occur due to the combinations of two or more different matters. The combination of different elements to form compounds is governed by some basic rules. These rules are called laws of chemical combination.
In this article, we are going to discuss the laws of chemical combination chemistry class 11. So let’s get started…
Laws of chemical combination
The combination of elements into compounds is governed by the following five basic laws.
Law of conservation of mass

This law was proposed by Antoine Lavoisier in 1789. He carried out careful experimental studies for combustion reactions and concluded that in all physical and chemical changes, there is no net mass change during the process. From this, he concluded that matter can neither be created nor destroyed. This is called the “law of conservation of mass”.
In any reaction mass of the reactant is equal to the mass of the product i.e mass can’t be created nor destroyed in any reaction.
This law formed the basis for several later developments in chemistry. In fact, this was the result of the accurate measurement of the masses of reactants and products and carefully designed experiments conducted by Lavoisier.
Read Also
Law of definite proportions

This law was proposed by a French chemist, Joseph Proust. He explained that a given compound always contains exactly the same weight proportion of elements. Proust worked with two samples of copper carbonate, one of which was natural and the other synthetic. He found that the composition of the elements it contained was the same for both samples, as shown below.
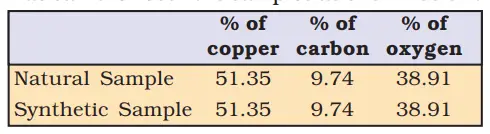
From this, he concluded that regardless of the source, a given compound always contains the same elements combined in the same mass ratio. The validity of this law was confirmed by several experiments. It is also sometimes referred to as the law of definite composition.
Law of multiple proportions
This law was proposed by Dalton in 1803. According to this law,
If two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
For example, hydrogen combines with oxygen to form two compounds, namely, water and hydrogen peroxide.$$ \underset{\rm 2g}{\rm Hydrogen} + \underset{\rm 16g}{\rm Oxygen} \rightarrow \underset{\rm 18g}{\rm Water}$$ $$ \underset{\rm 2g}{\rm Hydrogen} + \underset{\rm 32g}{\rm Oxygen} \rightarrow \underset{\rm 34g}{\rm Hydrogen\; peroxide}$$ Here, the masses of oxygen (i.e., $16\mathrm{~g}$ and $32 \mathrm{~g}$ ), which combine with a fixed mass of hydrogen (2g) gives a simple ratio, i.e., $16: 32$ or $1: 2$.
Read Also
- Measurement of physical properties of matter class 11 NCERT chemistry
- Importance of chemistry in our everyday life, class 11
Gay Lussac’s law of gaseous volumes

Gay Lussac
This law was established by Gay Lussac in 1808. He noticed that when gases combine or form in a chemical reaction, they do so in a simple volume ratio, provided that all gases are at the same temperature and pressure. In this way, 100 ml of hydrogen combine with 50 ml of oxygen to give 100 ml of water vapor. $$ \underset{\rm 100\; mL}{\rm Hydrogen} + \underset{\rm 50\;mL}{\rm Oxygen} \rightarrow \underset{\rm 100\;mL}{\rm Water}$$
Therefore, the combined volumes of hydrogen and oxygen (ie, 100 mL and 50 mL) have a simple 2:1 ratio. Gay Lussac’s discovery of the ratio of integers in the volume ratio is actually the law of definite proportions per volume. The above law of definite proportions referred to the mass. Gay Lussac’s law was adequately explained by Avogadro’s 1811 work.
Avogadro’s Law
In 1811, Avogadro suggested that equal volumes of all gases at the same temperature and pressure should contain the same number of molecules. Avogadro made a difference between atoms and molecules, which is understood nowadays. If we look again at the reaction of hydrogen and oxygen to create water, we see these two volumes of hydrogen combine with one volume of oxygen to give two volumes of water leaving no unreacted oxygen.
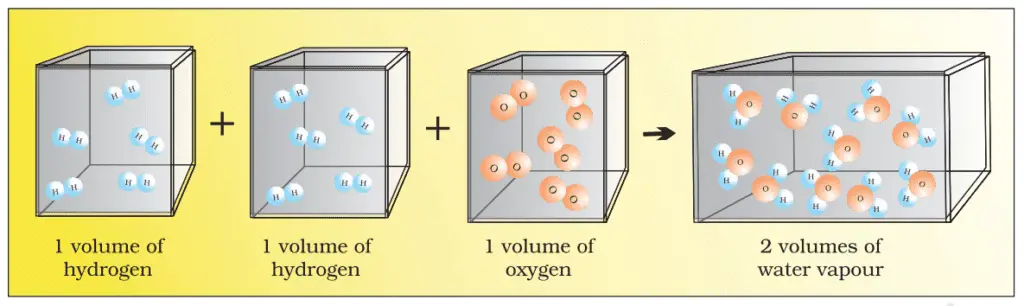

In the above figure, each box contains the same number of molecules. In fact, Avogadro could explain the above result considering that the molecules are polyatomic. If hydrogen and oxygen were considered diatomic now recognized, then the above results are easy to understand.
Read Also
- Matter | Nature of matter | classification of matter, class 11 | some basic concepts of chemistry
- Physical and chemical properties of matter chemistry class 11
However, Dalton and others believed at the time that atoms of the same kind cannot combine and that there are no oxygen or hydrogen molecules that contain two atoms. Avogadro’s proposal was published in the French Journal de Physique. Although correct, it did not receive much support.
Frequently Asked Questions – FAQs
What do the chemical combination laws explain?
The laws of chemical combination describe the basic principles followed by interacting atoms and molecules, interactions that can encompass a wide range of combinations that occur in a variety of ways. This incredible variety of interactions allows for an incredible variety of chemical reactions and compounds.
Name the five basic laws of chemical combination for elements and compounds.
The basic principles followed by interacting atoms and molecules are described by the laws of the chemical combination of elements and compounds. These interactions have numerous combinations that occur in different ways. The following are the five basic laws of chemical combination of elements and compounds:
1). Law of Conservation of Mass
2). Law of Definite Proportions
3). Law of Multiple Proportions
4). Gay Lussac’s Gas Volume
5). Law Avagadro’s Law of Chemical Combination
Who proposed the law of conservation of mass?
The Law of Conservation of Mass dates from Antoine Lavoisier’s 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction
Who gave the law of definite proportion?
The Law of Constant Composition, discovered by Joseph Proust, is also known as the Law of Definite Proportions.
What is a fixed ratio in chemistry?
Fixed ratios in chemistry are used to compare the amount of elements in a chemical formula.
Stay tuned with Laws Of Nature for more useful and interesting content.