The Cathode Tube Ray Experiment is a fascinating and groundbreaking discovery that revolutionized our understanding of the fundamental nature of matter. In this experiment, a cathode ray tube was used to investigate the properties of electrically charged particles, leading to the discovery of the electron and laying the foundation for modern physics.
The experiment, first conducted in the late 19th century by the British physicist J.J. Thomson, marked a crucial turning point in our understanding of the atomic world and paved the way for numerous technological advancements that we take for granted today.
In this article, we will discuss the cathode ray tube experiment by J.J Thomson, the components, procedure, observation, and conclusion. So hang tight as we delve deeper into the world of cathode rays and uncover the secrets that they hold.
What is a Cathode ray tube?
A cathode ray tube (CRT) is a vacuum tube that consists of an electron gun, which emits a stream of electrons, and a fluorescent screen, which displays the image produced by the electron beam.
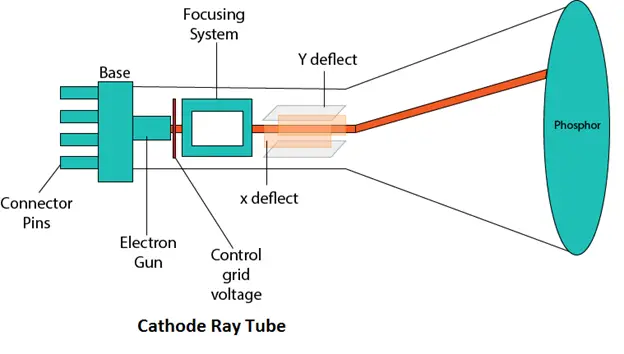
The tube is evacuated to a very low pressure, typically around $10^{-6}$ torr, to prevent the electrons from colliding with gas molecules in the tube. When a voltage is applied to the cathode, which is a negatively charged electrode, electrons are emitted from it and accelerated toward the anode, which is a positively charged electrode. As the electrons travel through the tube, they can be deflected by electric or magnetic fields, allowing them to be used for various purposes such as displaying images on a TV screen, measuring properties of charged particles, and even for particle accelerators.
What is a Cathode ray?
A cathode ray is a stream of electrons that are emitted from the cathode in a cathode ray tube. These electrons are negatively charged particles with a minimal mass compared to an atom’s. When a high voltage is applied between the cathode and anode in a cathode ray tube, electrons are emitted from the cathode and travel toward the anode. The resulting beam of electrons is known as a cathode ray.
Cathode ray tube components
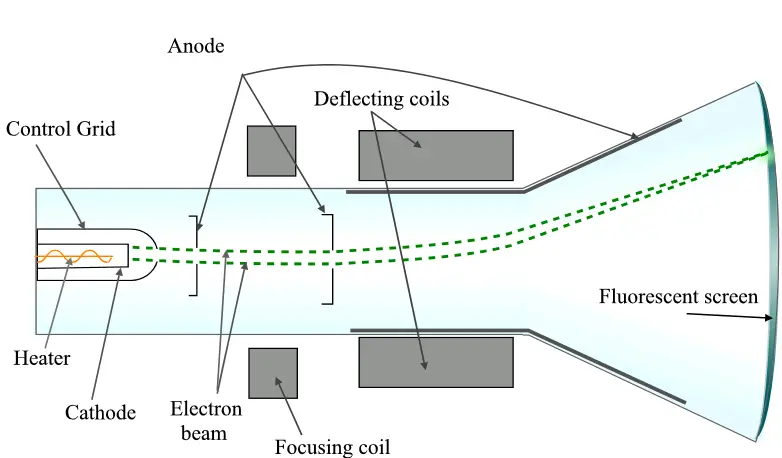
A cathode ray tube (CRT) typically consists of several key components, including:
- Cathode: The cathode is a negatively charged electrode that emits electrons when heated. It is usually made of a material that has a low work function, such as tungsten, which allows electrons to be emitted more easily.
- Anode: The anode is a positively charged electrode that accelerates the electrons emitted by the cathode. It is usually a metal plate with a small hole in the center to allow the electrons to pass through.
- Electron gun: The electron gun is a device that generates and focuses the electron beam. It typically consists of a cathode, a control grid, and a series of anodes and lenses that help to shape and focus the beam.
- Deflection system: The deflection system consists of one or more pairs of charged plates or coils that deflect the electron beam to produce the desired image on the screen.
- Fluorescent screen: The fluorescent screen is a coating of a material that emits light when struck by electrons. It is usually located at the end of the tube opposite the electron gun and deflection system.
- Vacuum pump: A vacuum pump is used to create and maintain a low-pressure environment inside the CRT, typically around $10^{-6}$ torr. This prevents the electrons from colliding with gas molecules in the tube and disrupting the electron beam.
These components work together to produce the characteristic electron beam and display images on the fluorescent screen.
Working of Cathode ray tube
The working of a cathode ray tube (CRT) involves several steps, as follows:
- Electrons are emitted from the cathode: When a voltage is applied to the cathode, it heats up and emits electrons. These electrons are negatively charged and are attracted to the positively charged anode.
- Electrons are accelerated by the anode: The anode is a positively charged electrode that attracts the negatively charged electrons emitted by the cathode. As the electrons are accelerated toward the anode, they gain kinetic energy.
- Electrons are focused by the electron gun: The electron gun consists of a series of anodes and lenses that help to shape and focus the electron beam. This beam of electrons is directed toward the fluorescent screen.
- Electrons are deflected by the deflection system: The deflection system consists of charged plates or coils that produce electric or magnetic fields that deflect the electron beam. By varying the strength and direction of these fields, the electron beam can be scanned across the screen to produce an image.
- Electrons strike the fluorescent screen: The fluorescent screen is coated with a material that emits light when struck by electrons. When the electrons in the beam strike the screen, they produce light that can be seen as an image.
Overall, the working of a cathode ray tube is based on the interaction between charged particles and electric or magnetic fields. By controlling the direction and strength of these fields, it is possible to produce and manipulate the electron beam and display images on the fluorescent screen.
Properties of cathode rays
Cathode rays are streams of electrons that are emitted from the cathode in a cathode ray tube. These electrons possess several properties, including:
- They are negatively charged: Cathode rays are composed of negatively charged electrons, which are fundamental particles of matter.
- They travel in straight lines: Cathode rays travel in straight lines in the absence of any electric or magnetic fields.
- They can be deflected by electric and magnetic fields: Cathode rays are deflected by electric and magnetic fields, indicating that they are charged particles.
- They can produce heat and light: When cathode rays collide with a solid surface or a gas, they can produce heat and light, similar to other charged particles.
- They have a very small mass: Cathode rays have a very small mass compared to the mass of an atom, indicating that they are subatomic particles.
- They have a high velocity: Cathode rays travel at very high velocities, typically approaching the speed of light.
Uses of Cathode Ray Tube
Cathode ray tubes (CRTs) have been widely used in various applications for over a century. Here are some of the common uses of cathode ray tubes:
- Television and computer displays: CRTs were widely used in older television and computer displays. The cathode ray tube inside the display projects an electron beam onto a phosphorescent screen, which creates the image that is visible on the screen.
- Medical equipment: Cathode ray tubes are still used in some medical equipment such as oscilloscopes, which are used to measure electrical signals in the body.
- Radar screens: Cathode ray tubes were used in early radar screens to display information about the location and movement of objects.
- Scientific experiments: CRTs are still used in scientific experiments to measure the properties of subatomic particles and to study the behavior of electrons in different conditions.
- Gaming machines: CRTs were used in the older arcades and gaming machines to display graphics and gameplay.
However, in recent years, CRTs have largely been replaced by other display technologies, such as LCD and LED screens, due to their smaller size, lower power consumption, and higher resolution.
J.J. Thomson Experiments – The Discovery of Electron
The Cathode ray experiment was the result of experiments using cathode ray tubes by an English physicist named J. J. Thomson. During his experiment, he discovered electrons, which is considered to be one of the most significant discoveries in the history of physics. He was even given the Nobel Prize in Physics for this discovery and his work on electrical conduction in gases.

However, when discussing the experiment, J. J. Thomson used a glass tube containing two pieces of metal as an electrode. High voltage was applied to the air inside the chamber, and electricity flowed through the air from the negative electrode to the positive electrode.
Read Also:
J.J. Thomson Cathode ray tube Experiment procedure
J.J. Thomson’s cathode ray tube experiments involved a series of steps and procedures that he followed to observe the behavior of electrons in the cathode ray tube. Here is a general outline of the procedure he used:
- A vacuum was created in the cathode ray tube to prevent the electrons from colliding with gas particles.
- A high voltage was applied between the cathode (negative electrode) and anode (positive electrode) in the tube using a power supply.
- Electrons were emitted from the cathode and traveled toward the anode, forming a beam of particles known as cathode rays.
- The cathode ray tube was equipped with various apparatus, such as electric and magnetic fields, which could be used to manipulate the electron beam.
- The behavior of the electron beam was observed by measuring the deflection of the beam as it passed through the electric and magnetic fields.
- The charge-to-mass ratio of the electrons was measured by analyzing the degree of deflection produced by the electric and magnetic fields and comparing it to the strength of the fields.
- Different gases were introduced into the cathode ray tube to observe the behavior of the electrons in the presence of different types of atoms or molecules.
Overall, Thomson’s cathode ray tube experiment procedure was a systematic approach that allowed him to carefully observe the behavior of electrons in the tube and draw conclusions about their properties and behavior.
Thomson’s First Cathode Ray Experiment
J.J. Thomson’s first cathode ray experiment, which he conducted in 1897, was a groundbreaking discovery that led to the identification of the electron as a fundamental particle of matter.
In this experiment, Thomson used a cathode ray tube to investigate the properties of electrically charged particles. He applied a high voltage between the cathode and the anode in the tube, creating a vacuum and allowing a beam of electrons to be emitted from the cathode.
Thomson then used electric and magnetic fields to measure the properties of the electron beam. By applying a magnetic field perpendicular to the beam, he observed that the beam was deflected, indicating that the electrons were negatively charged. Thomson also measured the charge-to-mass ratio of the electron by applying both electric and magnetic fields to the beam and found it to be much smaller than that of any previously known charged particle.
Based on these results, Thomson concluded that the electron was a fundamental particle of matter, and not a composite of smaller particles as was previously thought. His discovery revolutionized our understanding of the atomic world and laid the foundation for modern physics. Thomson was awarded the Nobel Prize in Physics in 1906 for his work on the electron.
Thomson’s Second Cathode Ray Experiment
J.J. Thomson’s second cathode ray experiment, which he conducted in 1899, was another important discovery that provided further evidence for the existence of the electron.
In this experiment, Thomson used a cathode ray tube similar to the one used in his first experiment, but he introduced a different type of gas into the tube. By changing the gas inside the tube, he was able to observe different types of cathode rays with different properties.
Thomson discovered that when a gas such as a hydrogen or helium was introduced into the cathode ray tube, a positively charged beam was emitted from the anode. This was in contrast to the negatively charged electron beam emitted from the cathode in his first experiment.
Thomson concluded that these positively charged particles, which he called “canal rays,” were the result of the ionization of the gas atoms in the tube. By measuring the charge-to-mass ratio of the canal rays, he found that they were positively charged and much heavier than electrons. This discovery provided further evidence for the existence of subatomic particles with different charges and masses.
Thomson’s work on canal rays and his identification of the electron as a fundamental particle revolutionized the field of atomic physics and laid the foundation for the development of modern particle physics.
Thomson’s Third Cathode Ray Experiment
J.J. Thomson’s third cathode ray experiment, conducted in 1904, was a continuation of his work on the properties of electrons and the nature of the electric charge.
In this experiment, Thomson used a cathode ray tube with a new design that allowed him to measure the velocity of the electrons in the beam more accurately. He also introduced a new type of gas into the tube, which allowed him to observe a wider range of electron energies.
Thomson observed that as the voltage applied to the cathode ray tube was increased, the electrons in the beam began to move faster and their paths became more curved. This indicated that the electrons were being accelerated by the electric field in the tube.
Thomson was also able to measure the charge-to-mass ratio of the electron with greater precision in this experiment, using a combination of electric and magnetic fields to deflect the electron beam. His measurements confirmed his earlier findings that the electron had a very small mass and a negative charge.
Thomson’s work on the properties of electrons and their behavior in electric and magnetic fields laid the groundwork for the development of electronic devices such as televisions, radios, and computers. His experiments also paved the way for the discovery of other subatomic particles such as protons and neutrons and contributed to our understanding of the structure of the atom.
Read Also:
- Atomic mass and molecular mass: chemistry class 11, NCERT
- John dalton’s atomic theory: postulates & limitations, class 11, NCERT
- Laws of chemical combination: chemistry class 11, NCERT
- Matter | Nature of matter | classification of matter, class 11 | some basic concepts of chemistry
- Stoichiometry and stoichiometric calculations class 11, NCERT
J.J. Thomson Cathode ray tube Experiment Observations
J.J. Thomson’s observations in his cathode ray tube experiments led to several important discoveries about the nature of the electron and its behavior in electric and magnetic fields. Some of his key observations include:
- The cathode ray emitted from the cathode in the tube was negatively charged and composed of particles with a minimal mass compared to that of the atom.
- The electron beam was deflected by both electric and magnetic fields, indicating that the electrons were charged particles.
- The charge-to-mass ratio of the electron was found to be much smaller than that of any previously known charged particle.
- When a different gas was introduced into the cathode ray tube, a positively charged beam was emitted from the anode. These positively charged particles were called “canal rays.”
- By measuring the charge-to-mass ratio of the canal rays, Thomson found that they were positively charged and much heavier than electrons.
Collectively, Thomson’s observations in his cathode ray tube experiments provided evidence for the existence of subatomic particles with different charges and masses and laid the foundation for the development of modern atomic and particle physics.
J.J. Thomson Cathode ray tube Experiment Conclusions
J.J. Thomson’s cathode ray tube experiments led to several important conclusions about the nature of the electron and its role in atomic structure. Some of his key conclusions include:
- The cathode rays emitted from the cathode in the tube were composed of negatively charged particles with a minimal mass compared to that of the atom.
- These negatively charged particles were later identified as electrons and were found to be fundamental particles of matter.
- The electron had a very small mass and a negative charge, as measured by the charge-to-mass ratio.
- Electrons could be accelerated by an electric field and deflected by a magnetic field, indicating their charge and the possibility of manipulating them with these fields.
- The discovery of positively charged “canal rays” when different gases were introduced into the tube provided further evidence for the existence of subatomic particles with different charges and masses.
Watch this video for more reference:
Frequently Asked Questions – FAQs
What particle did JJ Thomson discover?
J.J. Thomson discovered Electron.
Why cathode ray is called that?
The term “cathode ray” refers to a stream of electrons that are emitted by the negative electrode (cathode) in a vacuum tube. When an electric field is applied to the cathode, the electrons are accelerated toward a positively charged electrode (anode) in the tube.
The term “cathode ray” was coined by the English physicist William Crookes in the late 19th century. Crookes was conducting experiments on the properties of gases at low pressures using a vacuum tube and observed that a stream of particles (which he later identified as electrons) was emitted from the cathode when it was subjected to a high voltage.
The term “ray” was used to describe the stream of particles because it was thought to behave like a beam of light. The term “cathode” refers to the negative electrode that the electrons are emitted from.
So, in short, the term “cathode ray” describes the stream of electrons emitted from the cathode in a vacuum tube.
Is cathode ray positive or negative?
The cathode ray is negative. The cathode is the negatively charged electrode in a vacuum tube, and when a high voltage is applied to it, a stream of negatively charged particles (electrons) is emitted. These electrons are accelerated towards a positively charged electrode (anode) in the tube, creating the cathode ray.
How does the cathode ray experiment work?
The cathode ray experiment is a classic experiment in physics that was first conducted by J.J. Thomson in 1897. The experiment was designed to study the properties of the cathode ray, which is a stream of electrons emitted from the cathode in a vacuum tube. Here are the basic steps of the experiment:
1). A vacuum tube is constructed with two metal electrodes: a cathode (negative) and an anode (positive).
2). The tube is then evacuated to create a vacuum, which means that there is no air or other gas in the tube.
3). A high voltage is applied between the cathode and anode using a power supply. This causes electrons to be emitted from the cathode and travel towards the anode.
4). The cathode ray can be observed by placing a fluorescent screen at one end of the tube. When the electrons in the cathode ray hit the screen, they cause it to glow.
5). A pair of metal plates can be placed inside the tube, which are connected to a power supply. By varying the voltage applied to these plates, the path of the cathode ray can be bent or deflected. This allows the properties of the cathode ray to be studied in more detail.
Through this experiment, J.J. Thomson was able to determine that the cathode ray consisted of negatively charged particles (electrons) and that these particles had a minimal mass compared to atoms. This led to the discovery of the electron and the development of the modern model of the atom.
Which gas is used in the cathode ray tube?
The gas used in a cathode ray tube depends on the specific type of tube and its intended application. In some early cathode ray tubes, low-pressure gas such as air, nitrogen, or hydrogen was used.
However, in modern cathode ray tubes, which are used in televisions and computer monitors, the tube is typically filled with a vacuum, meaning that there is no gas present at all. This is because a vacuum allows the electrons in the cathode ray to travel more freely and without interference from gas molecules.
In some specialized applications, other gases may be used in cathode ray tubes. For example, neon is used in neon lamps, which use a small amount of gas to produce a glowing light when a high voltage is applied. However, this is not a traditional cathode ray tube application.
Stay tuned with Laws Of Nature for more useful and interesting content.