Introduction
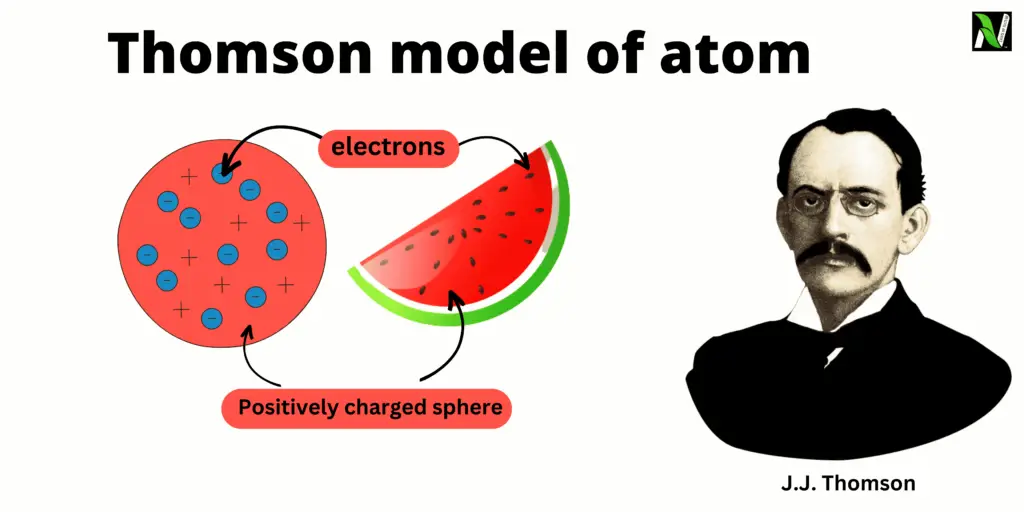
The Thomson Model Of Atom, proposed by the famous physicist J.J. Thomson in the late 19th century, marked a significant milestone in our understanding of atomic structure. In an era where the nature of atoms was still largely mysterious, Thomson’s model of atoms provided a groundbreaking explanation for the arrangement of particles within an atom. Also known as the “plum pudding model,” it depicted atoms as a positively charged sphere with embedded negatively charged electrons scattered throughout, much like plums in a pudding or seeds in a watermelon.
This revolutionary concept challenged the prevailing views of atomic theory at the time and laid the foundation for further advancements in our understanding of the microscopic world.
In this article, we will discuss the Thomson model of the atom (plum pudding model), its explanation, postulates, drawbacks, and significance. So join us on this journey as we unravel the fascinating story behind the Thomson Model of the Atom.
Thomson’s plum pudding model
The Thomson Plum Pudding Model, proposed by J.J. Thomson in the late 19th century, was an early atomic model that aimed to explain the structure of atoms. According to this model, atoms were depicted as a sphere of positive charge, like a pudding, with negatively charged electrons dispersed within it, resembling plums in the pudding.
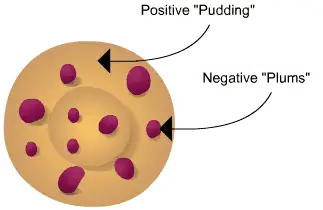
Thomson envisioned the atom as a uniform and positively charged substance. He believed that the positive charge was evenly distributed throughout the atom, and the negatively charged electrons were embedded within it. This arrangement helped explain why atoms were electrically neutral since the positive and negative charges balanced each other out.
The Plum Pudding Model represented an important advancement from past atomic theories that considered atoms as indivisible and indestructible particles. It presented a new understanding of atomic structure, suggesting that atoms were formed up of smaller subatomic particles.
Thomson’s model of atom explanation
Imagine you have a delicious plum pudding sitting in front of you. Now, let’s think about atoms. Back in the day, a brilliant scientist named J.J. Thomson came up with a fascinating idea called the Thomson Model of the Atom.
According to Thomson’s model, atoms are like tiny plum puddings! Picture the pudding as a big sphere made of positively charged stuff. It’s like a ball of positive charge.
Now, let’s add some plums to our pudding. These plums are tiny negatively charged particles called electrons. They are scattered all around the positive pudding sphere.
The interesting thing is that in Thomson model of atom, the positive charge is spread evenly throughout the atom, like the sweetness of the pudding. This balance of positive and negative charges made the atom electrically neutral.
Thomson’s Plum Pudding Model was a new way of thinking about atoms. It showed that atoms are not just solid and indivisible particles but actually made up of smaller particles.
However, as scientists continued to explore and experiment, they discovered that the Thomson Model wasn’t quite right. It couldn’t explain everything they observed. So, new models came along that provided a more accurate understanding of atoms.
But Thomson’s Plum Pudding Model was an important step in our journey of understanding atoms. It sparked curiosity and led to further discoveries that helped shape our modern understanding of the atomic world.
Read Also:
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Discovery of Electron class 11: chemistry, NCERT
- Discovery of Neutron class 11: history, properties, observations, and Conclusions
- Millikan oil drop experiment class 11: history, apparatus, procedure, observation
Postulates of Thomson model of atom
The Thomson Model of the Atom, also known as the Plum Pudding Model, proposed by J.J. Thomson, put forth a set of postulates to explain the structure of atoms. Below are the key postulates of the Thomson Model of atom:
- Atoms are composed of positively charged matter: According to Thomson’s model, atoms are made up of a positively charged substance, akin to a sphere. This positive charge is evenly distributed throughout the atom.
- Electrons are embedded in the atom: The model suggests that within the positively charged sphere, negatively charged particles called electrons are scattered or embedded randomly. These electrons balance out the positive charge, resulting in an electrically neutral atom.
- Electrons are indivisible: Thomson’s model did not consider the electrons as having any internal structure or being made up of smaller particles. In this model, electrons were considered fundamental particles.
- The atom is stable: The distribution of positive and negative charges in the atom according to the model allows it to remain stable. The positive charge within the atom keeps the negatively charged electrons attracted and bound within it.
- The size of the atom is finite: Thomson’s model did not provide a specific size for the atom but suggested that it occupies a finite amount of space.
These postulates formed the basis of the Thomson Model, providing an initial understanding of the atomic structure. However, subsequent experimental observations and advancements in atomic theory led to the development of more refined models, such as the Rutherford and Bohr models, which better explained the behavior and structure of atoms.
Drawbacks of Thomson model of atom
The Thomson Model of Atom, although groundbreaking at the time, had several drawbacks and limitations that became apparent as further research and experiments were conducted. Below are some of the main drawbacks of the Thomson Model of atom:
- Inability to explain the observed results of the gold foil experiment: The gold foil experiment, conducted by Ernest Rutherford, revealed that the majority of alpha particles passed straight through the foil, indicating that most of the atom is empty space. This observation contradicted the Thomson Model’s assumption of a uniform positive charge throughout the atom, as it would have caused greater deflections or collisions.
- Lack of an explanation for the stability of atoms: The Thomson Model failed to explain why atoms remain stable despite the negatively charged electrons being attracted to the positively charged region. According to classical electromagnetism, opposite charges should attract each other and cause the electrons to spiral into the positive sphere, leading to the collapse of the atom.
- Neglecting the existence of a nucleus: The Thomson Model did not account for the presence of a central, dense, and positively charged nucleus within the atom. Subsequent discoveries, such as Rutherford’s nuclear model, showed that the positive charge and most of the atom’s mass are concentrated in the nucleus, while electrons orbit around it.
- Lack of an explanation for spectral lines: The Thomson Model could not explain the discrete spectral lines observed in atomic emission and absorption spectra. It couldn’t account for the quantized energy levels of electrons and their transitions between these levels.
- Inadequate representation of atomic mass: The Thomson Model did not provide an accurate depiction of the distribution of atomic mass within the atom. It considered the positive charge to be spread uniformly throughout, whereas later models showed that the mass is concentrated in the nucleus.
Despite its drawbacks, the Thomson Model was important in laying the way for further advancements in atomic theory and our knowledge of atomic structure. It served as a foundation for future models that corrected its shortcomings and provided more precise explanations of the atom.
Significance of Thomsom model of atom
The Thomson Model of the Atom was significant in the development of atomic theory and scientific understanding. Below are some key points that highlight the significance of the Thomson Model of atom:
- Advancement of atomic theory: The Thomson Model was an advancement from previous ideas of atoms as indivisible, solid units. It first proposed that atoms are made up of smaller subatomic particles, specifically electrons, contained within a positively charged sphere. This was a significant step ahead in our understanding of atomic structure.
- Discovery of the electron: The Thomson Model played an important role in the discovery of the electron. J.J. Thomson measured the charge-to-mass ratio of the electron using cathode tube rays experiment, proving the existence of negatively charged particles within atoms. This discovery opened the way for further research into the nature of subatomic particles.
- Concept of atomic neutrality: The Plum Pudding Model explained the overall neutrality of atoms. The evenly distributed positive charge within the atom balanced out the negatively charged electrons, resulting in a neutral overall charge. This understanding of atomic neutrality became a fundamental concept in atomic theory.
- Influence on subsequent atomic models: The Thomson Model influenced and paved the way for the development of subsequent atomic models. It provided a starting point for further refinements, such as the Rutherford Model and the Bohr Model, which better explained the distribution of mass, the existence of a central nucleus, and the quantized energy levels of electrons.
- Bridging classical physics and quantum mechanics: The Thomson Model served as a bridge between classical physics and the emerging field of quantum mechanics. While it did not fully represent the complexities of atomic behavior, it laid the groundwork for future scientists to develop more comprehensive models that could explain phenomena at both macroscopic and microscopic scales.
- Historical significance: The Thomson Model represents an important milestone in the history of scientific discovery. It challenged existing beliefs about the nature of atoms and opened up new avenues of research and experimentation. Its drawbacks inspired further investigation, ultimately leading to the development of more accurate models that form the basis of our modern understanding of atomic structure.
Read Also:
- Atomic mass and molecular mass: chemistry class 11, NCERT
- John dalton’s atomic theory: postulates & limitations, class 11, NCERT
- Laws of chemical combination: chemistry class 11, NCERT
- Matter | Nature of matter | classification of matter, class 11 | some basic concepts of chemistry
- Stoichiometry and stoichiometric calculations class 11, NCERT
Frequently Asked Questions – FAQs
What is the JJ Thomson model of the atom?
According to this Thomson model, atoms were depicted as a sphere of positive charge, like a pudding, with negatively charged electrons dispersed within it, resembling plums in the pudding.
What are the features of the Thomson model of an atom?
The Thomson model of an atom has the following important features:
(i) An atom is a positively charged sphere with electrons embedded in it.
(ii) The negative and positive charge magnitudes are the same. As a result, the atom as a whole is electrically neutral.
What are the two postulates of the Thomson model of the atom?
The Thomson model of the atom had two main postulates:
1). Atoms are composed of positively charged matter: According to the Thomson model, atoms are made up of a sphere of positively charged matter. This positive charge is distributed uniformly throughout the atom.
2). Electrons are embedded in the atom: The model proposed that within the positively charged sphere, negatively charged electrons are embedded or scattered randomly. These electrons balance out the positive charge, resulting in an overall electrically neutral atom.
Why was Thomson’s model rejected?
Thomson’s model was rejected because it couldn’t explain the observations of the gold foil experiment, which showed that most alpha particles passed through the foil with only a few being deflected. This indicated that atoms have a dense, positively charged nucleus, which Thomson’s model did not account for.
What was missing from Thomson’s atomic model?
Thomson’s atomic model lacked a nucleus, protons, and neutrons.
Stay tuned with Laws Of Nature for more useful and interesting content.