A topic of class 11 chemistry NCERT, The “Discovery of the neutron”. In this article, we are going to discuss everything about the discovery of neutrons, so be with us through the journey of space and time, as we explore the remarkable story of the neutron discovery and the impact it has had on our world, So let’s get started…
Let’s start our discussion with the definition of neutron and its properties.
What is a neutron?
So what is a neutron? A neutron is a subatomic particle, symbol n or n⁰, which has a neutral charge, and a mass slightly greater than that of a proton. Both Protons and neutrons constitute the nuclei of atoms and are collectively known as nucleons.
Neutrons are made up of three quarks: two down quarks and one up quark. Down quarks have a charge of -1/3, and up quarks have a charge of 2/3. The total charge of a neutron is overall zero.
Neutrons are held together in the nucleus of an atom by a strong force. The strong force is a force that acts between all quarks, and it is much stronger than the electromagnetic force, which is the force that holds electrons in orbit around the nucleus.
Neutrons play a crucial role in determining the stability of atomic nuclei. Their presence helps balance the repulsive forces between positively charged protons, allowing the nucleus to hold together. Neutrons also contribute to the mass of the atom, but they have no direct involvement in chemical reactions.
Properties of neutrons
Below are some basic properties of neutrons:
- Charge: Neutrons have no charge. This means that they are not attracted to or repelled by electric fields.
- Mass: Neutrons have a mass of $1.674927498 \times 10^{-27}$ kg. This is slightly greater than the mass of a proton, which is $1.672621777 \times 10^{-27}$ kg.
- Radius: The radius of a neutron is estimated to be between 0.84 and $1.5 \times 10^{-15} m$. This is much smaller than the radius of an atom, which is typically on the order of $100 \times 10^{-12} m$.
- Half-life: The half-life of a neutron is 881.5±1.5 s. This means that if you have a group of 100 neutrons, after 881.5 seconds, there will be about 50 neutrons left. After 1763 seconds, there will be about 25 neutrons left, and so on.
- Composition: Neutrons are made up of three quarks: two down quarks and one up quark. Down quarks have a charge of -1/3, and up quarks have a charge of 2/3. The total charge of a neutron is therefore zero.
- Stability: Free neutrons are unstable and decay into protons, electrons, and antineutrinos. The half-life of a free neutron is about 15 minutes. This means that if you have a group of 100 neutrons, after 15 minutes, there will be about 50 neutrons left. After 30 minutes, there will be about 25 neutrons left, and so on.
- Interactions: Neutrons interact with other particles through the gravitational force, the weak force, and the strong force. The gravitational force is the weakest of the three forces, and it only plays a role in interactions between very massive objects. The weak force is responsible for radioactive decay, and it is much stronger than the gravitational force, but still weaker than the strong force. The strong force is the strongest of the three forces, and it is responsible for holding the nucleus of an atom together.
- Applications: Neutrons are used in a variety of applications, including nuclear power, nuclear weapons, and medical imaging. In nuclear power, neutrons are used to split atoms of uranium-235, which releases energy. This energy can then be used to generate electricity. In nuclear weapons, neutrons are used to create a chain reaction, which releases a large amount of energy in a very short period of time. In medical imaging, neutrons are used to create images of the body. This is done by using a device called a neutron camera.
History of the discovery of the neutron
Once upon a time, a clever scientist named James Chadwick wanted to solve a big puzzle. At that time, scientists knew that atoms had a small, dense part called the nucleus, which was made up of positively charged particles called protons. But they couldn’t figure out how the nucleus stayed together because the like-charged protons should repel each other and break the nucleus apart.

Chadwick decided to investigate this mystery. He used a special element called beryllium and shot tiny particles called alpha particles at it. Something interesting happened when he did this. He noticed a new kind of invisible radiation coming out of the beryllium. This radiation was different from the alpha particles and other types of radiation they knew about.
Chadwick studied this strange radiation carefully. He measured how it moved and how much energy it had. What he discovered was amazing! The radiation was made up of particles that were similar in size to protons, but they didn’t have any electric charge. They were like silent, neutral particles.
Chadwick called these new particles “neutrons” because they didn’t have any charge. They were the secret agents inside the nucleus, keeping everything stable and balanced.
This discovery was a big deal! It helped scientists understand why the nucleus didn’t fall apart and led to incredible advancements in nuclear power and medicine. Neutrons turned out to be super important in many areas of science.
So, thanks to James Chadwick’s curiosity and hard work, we now know about these special particles called neutrons that play a crucial role in the tiny world of atoms. They may be small, but their discovery had a huge impact on our understanding of the universe.
Read Also
- Discovery of proton class 11: chemistry NCERT
- Discovery of Electron class 11: chemistry, NCERT
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Charge-to-mass ratio of electron, chemistry class 11 NCERT
- Charge of electron, class 11: chemistry NCERT
Timeline of discovery of the neutron
The history of the discovery of neutrons involves the contributions of several scientists. Here’s a timeline of the discovery of neutrons:
- 1911: Ernest Rutherford proposed the nuclear model of the atom, suggesting that the positive charge and most of the mass of an atom are concentrated in a tiny, dense nucleus. However, the nature of the particles within the nucleus remained unclear.
- The 1920s: Researchers, including Rutherford himself, speculated about the existence of a neutral particle in the nucleus to explain its stability. However, experimental evidence was still lacking.
- 1930: Walther Bothe and Herbert Becker performed an experiment in which they bombarded beryllium with alpha particles and observed unexplained penetrating radiation. They speculated that this radiation might consist of neutral particles, but they couldn’t confirm it definitively.
- 1932: James Chadwick, a British physicist working at the Cavendish Laboratory in Cambridge, conducted his own experiments to investigate the mysterious radiation observed by Bothe and Becker.
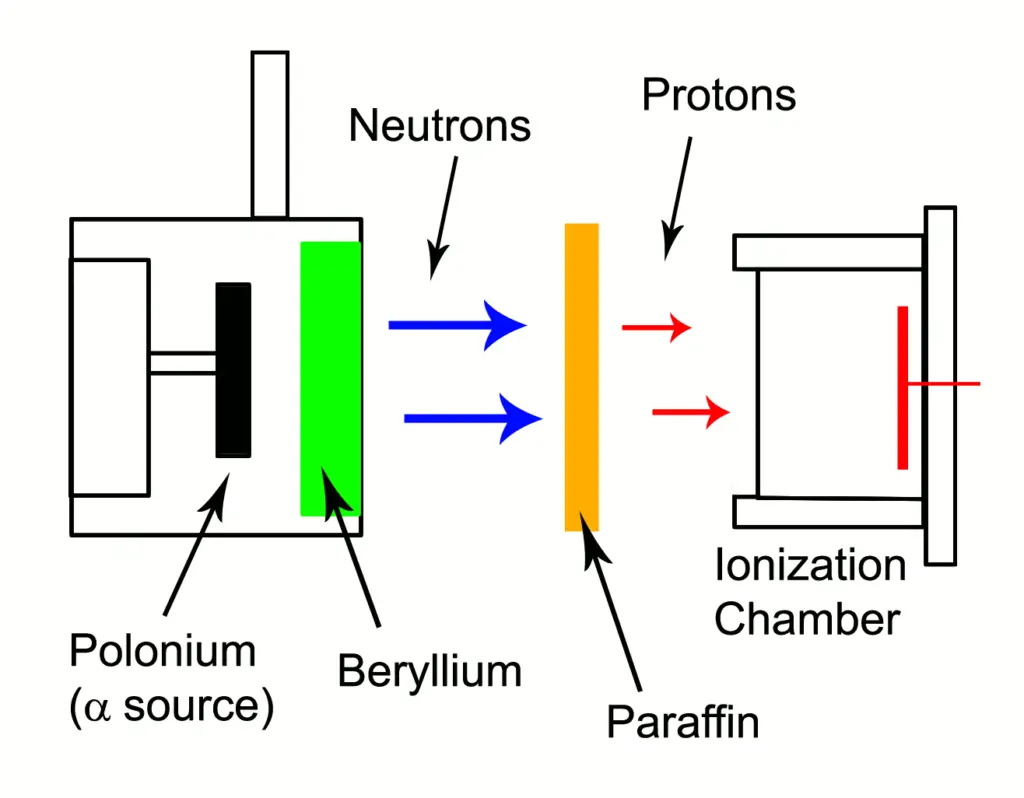
- Chadwick designed an experiment in which he placed a piece of beryllium inside a vacuum chamber and bombarded it with alpha particles from a radioactive source Polonium. He measured the radiation produced and observed its properties.
- Through careful measurements of the energy and scattering angles of the radiation, Chadwick concluded that it consisted of particles with a mass similar to that of protons but with no electrical charge. He named these particles “neutrons” to reflect their neutrality.
- Chadwick published his findings in February 1932 in the journal Nature, presenting strong evidence for the existence of neutrons. His discovery filled a crucial gap in understanding the atomic nucleus and explained its stability.
- James Chadwick’s discovery of the neutron was widely recognized and confirmed by subsequent experiments. He was awarded the Nobel Prize in Physics in 1935 for his fundamental discovery.
The discovery of neutrons by James Chadwick revolutionized our understanding of the atomic structure and paved the way for advancements in nuclear physics, nuclear energy, and numerous other fields. It provided a deeper understanding of the forces and interactions within atomic nuclei.
How Neutron was discovered?
The discovery of the neutron was made through experimental research conducted by James Chadwick in the early 1930s. Below is a detailed account of the experimental process:
- Chadwick began his investigations by building upon the work of other scientists, particularly Ernest Rutherford’s studies on atomic structure and the nucleus. Rutherford suggested the existence of a neutral particle in the nucleus to account for its stability.
- Chadwick designed an experiment to investigate the radiation produced when alpha particles (helium nuclei) were fired at various elements. He focused on beryllium, as it had shown interesting results in previous experiments.
- Chadwick placed a piece of beryllium inside a vacuum chamber and bombarded it with alpha particles from a radioactive source. He observed that in addition to the expected alpha particles and beta particles (electrons), a new type of radiation was being emitted.
- Chadwick conducted several measurements and analyses of this mysterious radiation. He observed that it possessed properties different from those of alpha and beta particles. It could penetrate materials more effectively than expected, and it did not carry an electric charge.
- By carefully measuring the energy and scattering angles of the radiation, Chadwick deduced that it consisted of particles with a mass similar to that of protons but with no electrical charge. This implied that he had discovered a new neutral particle.
- In 1932, Chadwick published his groundbreaking findings in the journal Nature, presenting strong evidence for the existence of neutrons. He named the new particle “neutron” to reflect its neutrality.
Read Also:
- Percentage composition chemistry class 11: formula, definition, examples, NCERT
- Mole concept class 11 | definition, formula & solved examples, NCERT
- Uncertainty in measurement chemistry, class 11 NCERT
- Empirical formula & molecular formula: chemistry class 11
Discovery of neutron: Chadwick Observations
James Chadwick made several important observations during his experiments that led to the discovery of neutrons. Here are some main observations made by Chadwick:
- Penetrating Radiation: Chadwick observed that when he bombarded a piece of beryllium with alpha particles, a new type of radiation was emitted. This radiation had the ability to penetrate materials more effectively than alpha or beta particles, which suggested that it possessed high energy and no electric charge.
- Mass Similar to Protons: By carefully measuring the energy and scattering angles of the radiation, Chadwick determined that the particles responsible for the radiation had a mass similar to that of protons. This indicated that they were not electrons or alpha particles but instead a new type of particle.
- Electrically Neutral: Chadwick’s most significant observation was that the particles causing the radiation were electrically neutral. This was determined by observing that the radiation was not deflected by electric or magnetic fields, which indicated the absence of an electric charge.
Discovery of neutron: Chadwick Conclusions
After the experiment of bombarding alpha particles from a radioactive source (Polonium) on the beryllium surface. He concluded various things after his observations. Below are some of the specific conclusions that Chadwick made about neutrons:
- Neutrons are neutrally charged. This means that they have no electric charge, which is why they are not attracted to or repelled by electric fields.
- Neutrons have a mass slightly greater than that of a proton. This means that they are slightly heavier than protons, which have a mass of $1.672621777 \times 10^{-27}$ kg.
- Neutrons are unstable and decay into protons, electrons, and antineutrinos. This means that they do not last forever, but instead break down into other particles. The half-life of a neutron is about 15 minutes, which means that if you have a group of 100 neutrons, after 15 minutes, there will be about 50 neutrons left. After 30 minutes, there will be about 25 neutrons left, and so on.
- Neutrons are held together in the nucleus of an atom by a strong force. The strong force is a force that acts between all quarks, and it is much stronger than the electromagnetic force, which is the force that holds electrons in orbit around the nucleus.
- Neutrons are used in a variety of applications, including nuclear power, nuclear weapons, and medical imaging. In nuclear power, neutrons are used to split atoms of uranium-235, which releases energy. This energy can then be used to generate electricity. In nuclear weapons, neutrons are used to create a chain reaction, which releases a large amount of energy in a very short period of time. In medical imaging, neutrons are used to create images of the body. This is done by using a device called a neutron camera.
Chadwick’s conclusions about neutrons were a major breakthrough in physics. They helped scientists to understand the structure of the atom and the forces that hold it together. They also led to the development of nuclear power and nuclear weapons.
Significance of neutrons
The discovery of the neutron by James Chadwick has immense significance in the field of physics and has had far-reaching implications in various areas. Here are some of the key significances of the neutron:
- Nuclear Stability: The neutron is a fundamental building block of atomic nuclei. Its discovery explained the stability of atomic nuclei, as the repulsive forces between the positively charged protons in the nucleus are counterbalanced by the presence of neutral neutrons. This understanding of nuclear stability is crucial for our understanding of the physical properties and behavior of matter.
- Nuclear Reactions and Energy: Neutrons play a pivotal role in nuclear reactions. They can induce nuclear fission, the splitting of atomic nuclei, which releases an enormous amount of energy. This discovery was essential for the development of nuclear power and nuclear weapons. Neutrons are also used in various research applications, such as neutron scattering and neutron activation analysis.
- Particle Physics: Neutrons are one of the fundamental particles in the Standard Model of particle physics. Their discovery contributed to our understanding of the fundamental particles that make up the universe. Neutrons, along with protons and electrons, form the basis of atomic and subatomic structures.
- Isotopes and Radioactivity: Neutrons have a crucial role in the formation of isotopes. By varying the number of neutrons in an atomic nucleus, different isotopes of an element can be created. This is significant in various fields, including medicine (radioisotopes for diagnostics and treatment) and agriculture (radiation for plant breeding and food preservation).
- Fundamental Forces: The discovery of neutrons deepened our understanding of the fundamental forces that govern matter. Neutrons interact with other particles through the strong nuclear force, one of the four fundamental forces of nature. The study of neutron interactions and the properties of the strong nuclear force have contributed to our knowledge of particle physics and the structure of matter.
Frequently Asked Questions – FAQs
What is a neutron?
A neutron is a subatomic particle, symbol n or n⁰, which has a neutral charge, and a mass slightly greater than that of a proton.
Who discovered neutrons?
James Chadwick discovered neutrons in 1932.
Is neutron discovered by Goldstein?
No, the neutron was discovered by James Chadwick. Proton was discovered by E Goldstein.
How did Chadwick discover neutron?
James Chadwick discovered the neutron in 1932 by bombarding beryllium with alpha particles. This caused the beryllium to emit a neutral particle with about the same mass as a proton. Chadwick named this particle the neutron.
Where was the neutron discovered?
Neutron was discovered by James Chadwick at Cavendish Laboratory in Cambridge.
Why was paraffin wax used in neutron discovery?
Paraffin wax was used in the discovery of the neutron because it slows down neutrons without absorbing them. This makes it easier to detect them.
Stay tuned with Laws Of Nature for more useful and interesting content.