Have you ever wondered about the fundamental building blocks of matter? At the heart of atoms, we have electrons, tiny negatively charged particles that orbit around the positively charged nucleus. But have you ever stopped considering how we know that electrons carry a negative charge? And why is this charge so crucial for understanding the behavior of matter and electricity?
In this post, we’ll dive into the fascinating world of electron charge, exploring the history of its discovery, the experiments that have helped us understand it, and the crucial role it plays in modern technology. So buckle up and get ready to learn about one of the most fundamental concepts in physics: the charge of electrons.
What is an electron?
An electron is a subatomic particle that carries a negative electric charge. It is one of the fundamental particles that make up atoms, along with protons and neutrons. Electrons are much lighter than protons and neutrons, with a mass of approximately 1/1836th that of a proton.
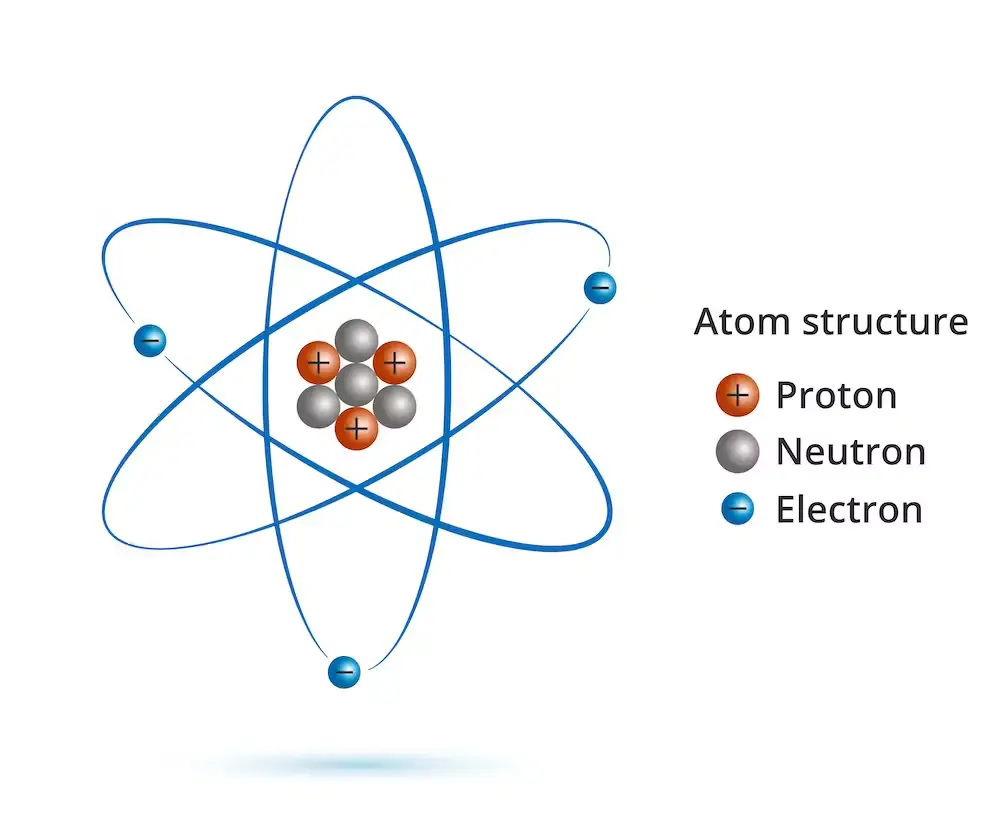
They are found outside of the atomic nucleus and orbit around it in shells or energy levels. The behavior of electrons is essential for understanding the properties and behavior of atoms, molecules, and the materials that make up our world. Electrons are also crucial for the functioning of modern technology, from electronics and computers to medical imaging and telecommunications.
History and discovery of the electron
The discovery of the electron is a fascinating story that spans several decades and involved many scientists. The first clues that led to the discovery of the electron came from experiments conducted in the late 1800s on cathode rays, streams of particles that were observed to flow from the negative electrode in a vacuum tube.

In 1897, J.J. Thomson, a British physicist, conducted a series of experiments using cathode ray tubes and discovered that these rays were made up of negatively charged particles much smaller than atoms. He called these particles “corpuscles,” but they later became known as electrons.
Thomson’s experiments showed that electrons were negatively charged and had a very small mass compared to atoms. This discovery challenged the prevailing view of the time that atoms were indivisible and opened up a new field of study in physics.
Later experiments by other scientists, such as Robert Millikan’s famous oil drop experiment in 1909, helped to determine the charge and mass of electrons more accurately and further confirmed their existence.
The discovery of the electron revolutionized our understanding of matter and paved the way for the development of modern electronics, which rely heavily on the behavior and manipulation of electrons. Thomson’s work earned him the Nobel Prize in Physics in 1906, and his discovery remains one of the most significant contributions to the field of physics to this day.
What is charge of electron?
The charge of the electron is a fundamental property of this subatomic particle. Electrons carry a negative charge of $-1.602 \times 10^{-19}$ Coulombs (C), which is the basic unit of electric charge in the International System of Units (SI).
The concept of electric charge was first introduced by the French physicist Charles-Augustin de Coulomb in the late 18th century. Coulomb’s law states that like charges repel each other, while opposite charges attract. The negative charge of the electron is what causes it to be attracted to the positively charged atomic nucleus, and keeps it in orbit around the nucleus.
The charge of the electron is crucial for understanding the behavior of matter and electricity. For example, when electrons move from one place to another, an electric current is created, which can be used to power electronic devices such as lights, computers, and smartphones. The charge of the electron also plays a critical role in chemical reactions, where electrons are exchanged between atoms to form new molecules.
Read Also:
- Atomic mass and molecular mass: chemistry class 11, NCERT
- John dalton’s atomic theory: postulates & limitations, class 11, NCERT
- Laws of chemical combination: chemistry class 11, NCERT
- Matter | Nature of matter | classification of matter, class 11 | some basic concepts of chemistry
- Stoichiometry and stoichiometric calculations class 11, NCERT
How did we know that electrons carry a negative charge?
The discovery that electrons carry a negative charge was made by the English physicist J.J. Thomson in the late 19th century through his experiments on cathode rays.
Cathode rays are streams of electrons that are emitted from the negatively charged electrode (cathode) in a vacuum tube when a high voltage is applied to it. Thomson conducted a series of experiments in which he passed cathode rays through a pair of parallel metal plates, which were connected to a battery to create an electric field between them.
Thomson observed that the cathode rays were deflected by the electric field and that the direction of deflection depended on the polarity of the electric field. In particular, he found that the cathode rays were deflected towards the positively charged plate, indicating that they carried a negative charge.
To confirm this result, Thomson repeated the experiment using a different type of cathode ray tube that had a fluorescent screen on one end. When the cathode rays struck the screen, they produced a visible glow. By measuring the angle of deflection and the strength of the electric field, Thomson was able to calculate the ratio of the charge to the mass of the cathode ray particles, which he found to be the same for all types of cathode rays.
Thomson’s experiments established that cathode rays were composed of negatively charged particles, which he called “corpuscles” (later known as electrons). This discovery was a major breakthrough in the understanding of the atomic structure and the nature of electricity and paved the way for further research on subatomic particles.
Mass and charge of electron
The mass and charge of an electron are two fundamental properties that define this subatomic particle. The current best-known values for these properties are:
- Charge of an electron: The electron carries a negative charge of $-1.602 \times 10^-19$ Coulombs (C). This is the basic unit of electric charge in the International System of Units (SI).
- Mass of an electron: The electron has a mass of $9.109 \times 10^-31$ kilograms (kg). This is approximately $1/1836th$ of the mass of a proton.
The charge and mass of the electron were first measured by the English physicist J.J. Thomson in his experiments on cathode rays in the late 19th century. Thomson’s discovery of the electron revolutionized our understanding of the atomic structure and paved the way for modern physics and technology.
The small mass and negative charge of the electron make it particularly important in chemical reactions and electrical phenomena. Electrons are responsible for the formation of chemical bonds between atoms, and the movement of electrons in wires and circuits is the basis for electric power and electronic devices.
Charge of electron, proton, and neutron
Electrons, protons, and neutrons are subatomic particles that makeup atoms. Each of these particles carries a different charge, as follows:
- Electron: Electrons carry a negative charge of $-1.602 x 10^-19$ Coulombs (C).
- Proton: Protons carry a positive charge equal in magnitude to the charge of an electron, which is $+1.602 \times 10^-19$ C.
- Neutron: Neutrons carry no electric charge, and are considered to be electrically neutral.
What is the specific charge of electron?
The specific charge of an electron is a physical constant that represents the ratio of the electron’s charge to its mass. Specifically, it is defined as the electric charge per unit mass of an electron and is expressed in units of coulombs per kilogram (C/kg).
$$\text{Specific Charge}=\frac{\text{Electronic Charge}}{\text{Mass}}$$
The specific charge of the electron was first measured by J.J. Thomson in his experiments on cathode rays, where he was able to determine the charge and mass of the electron separately. Thomson found that the specific charge of the electron was approximately $1.76 \times 10^{11} C/kg$.
The specific charge of the electron is a crucial property for understanding the behavior of electrons in electric and magnetic fields. It allows us to predict the trajectory of an electron in a given field and is also used in various applications such as medical imaging and particle accelerators.
It’s worth noting that the specific charge of the electron is much larger than that of other subatomic particles such as protons and neutrons. This is due to the fact that electrons have a much smaller mass than these particles, which means that they are much more affected by electric and magnetic fields.
Read Also:
- Charge-to-mass ratio of electron, chemistry class 11 NCERT
- Cyclotron class 12, definition, working principle, uses, advantages and limitations
- Different types of the cyclotron
Charge of electron in eV
The charge of the electron can also be expressed in electronvolts (eV), which is a unit of energy commonly used in physics. To convert the electron charge from Coulombs to eV, we can use the relationship:
$$1 eV = 1.602 \times 10^{-19} J$$
where J stands for Joules, the SI unit of energy.
Therefore, we can calculate the charge of an electron in eV by dividing the electron charge in Coulombs by the charge of one electron in Coulombs, and multiplying by the conversion factor to eV:
- Charge of electron in eV = (-1.602 x 10^-19 C / 1 electron) x (1 eV / 1.602 x 10^-19 J)
- Charge of electron in eV = -1 eV
Thus, the charge of an electron is equivalent to -1 eV. It’s worth noting that this value represents the amount of energy required to move one electron from one point to another against an electric potential difference of one volt.
What is the significance of charge of electron?
The charge of the electron is a fundamental property that plays a crucial role in many areas of physics, chemistry, and technology. Here are some of the key ways in which the charge of the electron is significant:
- Atomic structure: The negative charge of the electron plays a crucial role in determining the structure of atoms. Electrons occupy discrete energy levels around the nucleus of an atom, and their interactions with other electrons and the positively charged nucleus determine the chemical properties of elements.
- Chemical reactions: The movement of electrons between atoms is the basis for chemical reactions, and the electron’s negative charge is critical for the formation of chemical bonds.
- Electricity and electronics: The movement of electrons in wires and circuits is the basis for electric power and electronic devices. The properties of semiconductors, which form the basis of modern electronics, are largely determined by the behavior of electrons.
- Particle physics: The charge of the electron is a fundamental property that determines its interactions with other particles. It is also used as a reference point for defining the charges of other particles, such as protons and neutrons.
Read Also:
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Discovery of Electron class 11: chemistry, NCERT
- Charge-to-mass ratio of electron, chemistry class 11 NCERT
Frequently Asked Questions – FAQs
What is the charge in an electron?
The charge in an electron is a fundamental property that describes its negative electrical charge, which plays a crucial role in determining the behavior of matter at the atomic and subatomic levels.
What is the charge of a proton?
Protons carry a positive charge equal in magnitude to the charge of an electron, which is $+1.602 \times 10^{-19}$ C
Is electron positive or negative?
Electron is electrically negative.
What is the smallest unit of charge?
The smallest unit of charge is the charge of a single electron, which is approximately equal to $-1.602 \times 10^{-19}$ coulombs.
Which is the largest unit of charge?
The largest unit of charge that is commonly used is the coulomb (C), which is equivalent to the charge transported by a current of one ampere in one second.
One coulomb is a relatively large unit of charge, and charges are often measured in smaller units such as micro coulombs (μC) or nano coulombs (nC).