The Rutherford Atomic Model, proposed by scientist Ernest Rutherford in 1911, is a simple and fundamental concept in understanding the structure of an atom. In this model, Rutherford explained that an atom is a mostly empty space with a small, positively charged nucleus at its center.
Around this nucleus, negatively charged electrons orbit in set, predictable paths. This atomic model was a significant development in atomic physics, paving the way for more advanced atomic theories and models like the Bohr model.
In this article, we will discuss Rutherford’s atomic model, postulates, observations, and limitations in detail. So to know everything about Rutherford’s atomic model, stay with the article till the end.
In what way is rutherfords atomic model different from that of Thomson’s model of the atom
The Rutherford Atomic Model and Thomson Model of the atom are two early concepts that aimed to explain the structure of an atom. Here are the key differences between these:
- Thomson’s Plum Pudding Model: Proposed by J.J. Thomson in 1897, this model suggests that an atom is composed of a positively-charged “sphere” and negatively-charged electrons distributed uniformly within it, akin to plums embedded in a pudding. This model did not provide any details about the atomic nucleus.
- Rutherford’s Atomic Model: Ernest Rutherford, in 1911, introduced a new atomic model which established the presence of a small, dense, positively charged nucleus at the center of an atom. In this model, negatively charged electrons orbit the nucleus, and most of the atom is empty space.
The main difference between the Thomson and Rutherford models is the presence and understanding of the atomic nucleus. Thomson’s model does not account for a nucleus, while Rutherford’s model introduces the concept of a central nucleus and the orbital paths of electrons around it. This fundamental difference led to the development of more advanced atomic theories, such as the Bohr model, which further refined our understanding of atomic structure.
Rutherford atomic model class 11
Rutherford’s Atomic Model, also known as the nuclear model, was proposed by Ernest Rutherford after his gold foil experiment in 1911. This model is centered around two main components: the nucleus and the atom’s remaining space occupied by electrons.
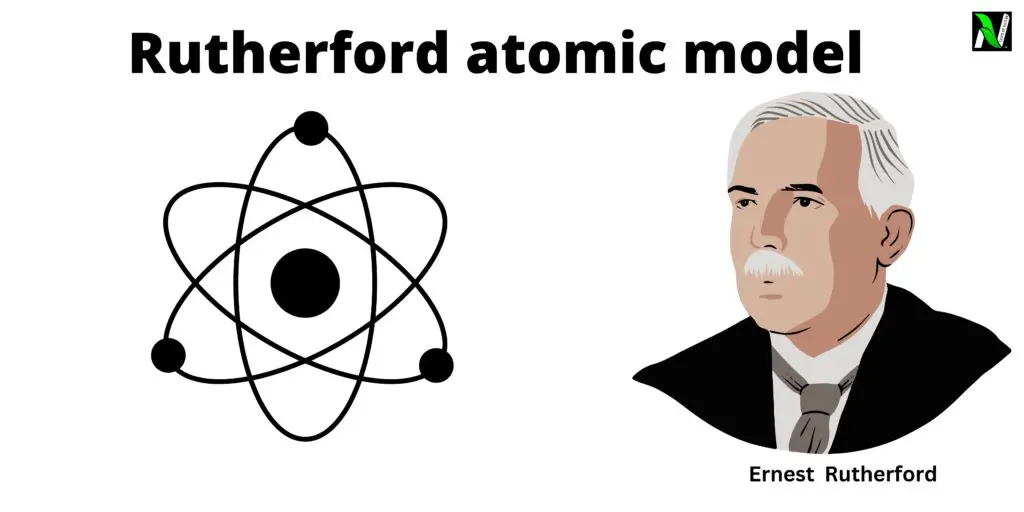
In Rutherford’s model, he proposed that an atom’s nucleus is both tiny and heavy, containing all the protons and neutrons, which make up almost all of the atom’s mass. The nucleus is also positively charged due to the presence of protons. Surrounding the nucleus is a vast empty space in which negatively charged electrons are found. These electrons orbit the nucleus at varying distances.
Rutherford’s atomic model replaced Thomson’s plum pudding model and introduced the concept of a central nucleus with orbiting electrons, fundamentally changing our understanding of atomic structure.
Explain Rutherford’s atomic model
Rutherford’s atomic model, also known as the nuclear model, was developed by Ernest Rutherford based on the findings of his gold foil experiment in 1911. This model explains the structure of an atom with two main components: the nucleus and the space surrounding it, occupied by electrons.
The central idea of Rutherford’s model is that the nucleus of an atom is small and heavy, containing positively charged protons and, later found to contain neutral particles called neutrons. The nucleus is responsible for most of the atom’s mass. Negatively charged electrons occupy the space around the nucleus, moving in orbits at varying distances.
Although Rutherford’s model was revolutionary for its time and significantly improved our understanding of atomic structure, it had some limitations such as not explaining the stability of atoms, electromagnetic radiation while electrons orbit, and the arrangement of electrons around the nucleus. These limitations were later addressed by the Bohr model, which incorporated quantum theory to provide a more accurate depiction of atomic structure.
Rutherford atomic model diagram
The diagram for Rutherford atomic model is given below:
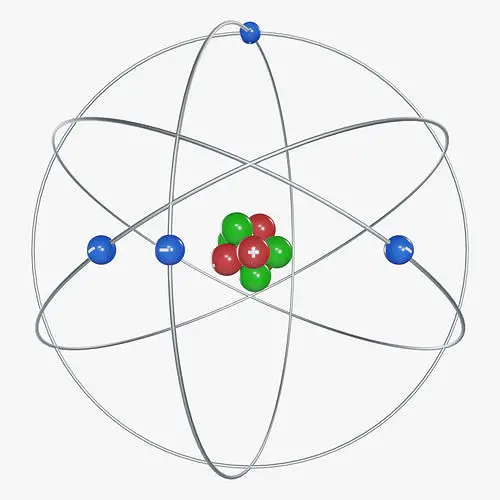
Postulates of Rutherford’s atomic model
The main postulates of Rutherford’s Atomic Model are as follows:
- An atom consists of two parts: the nucleus and the extra-nuclear region.
- The nucleus is a small, dense region at the center of the atom containing all the mass and the entire positive charge, in the form of protons and neutrons.
- The extra-nuclear region is the space surrounding the nucleus, which is mostly empty.
- Electrons, which are negatively charged particles, are present in the extra-nuclear region and orbit the nucleus at varying distances.
- The positive charge of the nucleus and the negative charge of the electrons balance each other, making the atom neutral overall.
Limitations of Rutherford’s atomic model
Rutherford’s atomic model, also known as the planetary model, was proposed by Ernest Rutherford in 1911. Although it was a groundbreaking model that contributed significantly to our understanding of the atom, it had several limitations. Some of the key limitations of Rutherford’s atomic model are given below:
- Lack of Stability: According to Rutherford’s model, electrons move around the nucleus like planets around the sun. However, this model couldn’t explain why atoms don’t collapse. The model suggested that electrons would lose energy and spiral into the nucleus, causing atoms to fall apart.
- Failure to Explain Line Spectra: Rutherford’s model couldn’t explain why atoms emit or absorb light at specific wavelengths instead of a continuous range of colors. It couldn’t account for the observed patterns in the colors of light emitted or absorbed by atoms.
- Absence of Electron Spin: Rutherford’s model didn’t include the idea that electrons have a property called spin. Spin is important for understanding how atoms behave, and it affects things like the arrangement of elements in the periodic table and the magnetic properties of atoms.
- Arrangements of electrons: Rutherford’s model did not say anything about how electrons are arranged around the nucleus, which made his theory incomplete.
- Neglect of Quantum Mechanics: Rutherford’s model was developed before scientists understood quantum mechanics, which describes how tiny particles like electrons behave. Quantum mechanics introduced the idea that electrons can exist in wave-like forms and have uncertain positions and energies. Rutherford’s model didn’t take these quantum principles into account.
- Lack of Explanation for Chemical Bonding: Rutherford’s atomic model couldn’t explain how atoms join together to form chemical bonds. It didn’t account for how electrons are shared or transferred between atoms, which is important for understanding how different elements combine to form compounds.
Read Also:
- Thomson model of atom: postulates, drawbacks, & significance, class 11
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Discovery of Electron class 11: chemistry, NCERT
- Discovery of proton class 11: chemistry NCERT
- Discovery of Neutron class 11: history, properties, observations, and Conclusions
- Millikan oil drop experiment class 11: history, apparatus, procedure, observation
Frequently Asked Questions – FAQs
What was the major finding of Rutherford’s gold foil experiment?
The gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus, leading to the proposal of the nuclear model of the atom.
How did Rutherford’s atomic model differ from Thomson’s plum pudding model?
Thomson’s plum pudding model described atoms as a uniform distribution of positive charge with embedded electrons, whereas Rutherford’s atomic model proposed a central nucleus containing the positive charge with electrons orbiting around it.
What kind of experiment did Rutherford perform?
Rutherford performed an alpha scattering experiment, where he bombarded alpha particles onto a thin gold foil and observed their paths using a fluorescent screen.
What was the specialty of Rutherford’s atomic model?
The specialty of Rutherford’s atomic model is that it introduced the concept of a small, dense, positively charged nucleus at the center of the atom, with electrons orbiting around it. This model replaced the earlier plum pudding model and changed our understanding of atomic structure by showing that atoms are mostly empty spaces with a concentrated positive charge in the nucleus.
What were the limitations of Rutherford’s atomic model?
Rutherford’s model had several limitations, including not explaining the stability of atoms, electromagnetic radiation, arrangement of electrons around the nucleus, and spectral lines. These limitations led to the development of more advanced models like the Bohr model, which incorporated quantum theory.
Stay tuned with Laws Of Nature for more useful and interesting content.