Let’s dive into the fascinating world of atomic physics and explore the Bohr Atomic Model—a really important idea that changed the way we see atoms. Niels Bohr, a smart scientist, came up with this model a long time ago, in the early 1900s.
It helped us understand how atoms work and totally transformed our understanding of chemistry and physics. So, let’s go on a fun journey to learn all about Bohr’s model, what it’s based on, and how it still matters today.
Bohr’s model was a big discovery and made a huge impact on science. But, like everything, it also had some limits. These limits led to more amazing discoveries and a new way of understanding atoms called Quantum Mechanics. So, get ready to uncover the exciting story of Bohr’s work and how it changed our understanding of the super tiny atomic world.
1. The Historical Background of Bohr Atomic Model
Historical Background of the Bohr Atomic Model
The atomic theory had been a topic of scientific investigation for centuries before Niels Bohr proposed his model of the atom. However, before the 20th century, most of these theories were based on philosophical speculation rather than empirical evidence. The advent of quantum mechanics dramatically changed the discourse on atomic structure, providing a more sound theoretical foundation.
In the late 19th and early 20th century, key experimental revelations were reshaping the understanding of the atom. The discovery of the electron by J.J. Thompson in 1897, followed by Ernest Rutherford’s atomic model in 1911, laid the groundwork for Bohr’s revolutionary conception.
Read Also
- Uncertainty in measurement chemistry, class 11 NCERT
- Laws of chemical combination: chemistry class 11, NCERT
- John dalton’s atomic theory: postulates & limitations, class 11, NCERT
Bohr’s Academic Journey
Niels Bohr was born in Copenhagen, Denmark, in 1885. He studied physics at the University of Copenhagen and received his Ph.D. in 1911. That same year, he went to England to work in the laboratory of J.J. Thompson, the discoverer of the electron. But he was more intrigued by Rutherford’s nuclear atomic model proposed in the same year, so he moved to Manchester to work in Rutherford’s laboratory.

In 1890, the Bohr family posed for a photograph at Nærumgaard, the summer residence of the Adler family. Among them were Christian Bohr, Ellen Bohr (née Adler), and their three children: Jenny Bohr, Niels Bohr, and Harald Bohr. In the picture, Niels Bohr can be seen standing behind his sister on the right. source: https://nbi.ku.dk
Bohr found Rutherford’s model intriguing because it proposed that the atom consisted of a positively charged nucleus surrounded by negatively charged electrons. However, using classical physics, Bohr noticed a problem in this model. If classical physics was to be believed, electrons orbiting the nucleus would radiate energy and eventually spiral into the nucleus. Hence, the atom would be unstable.
Bohr’s Revolutionary Proposal
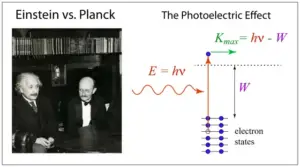
Bohr proposed his atomic model in 1913. He suggested that electrons could occupy only certain stable orbits around the nucleus, where they would not radiate energy. These orbits corresponded to specific energy levels, which could be calculated using Planck’s quantum theory. Furthermore, if an electron absorbed energy, it could jump to a higher energy level, and when it ‘fell’ back to its original level, it would emit energy in the form of light. This explained the line spectra observed in gas discharge tubes.
In essence, the Bohr atomic model successfully blended classical physics and quantum theory to explain the stability of the atom and atomic spectra – two phenomena that were inexplicable under the previous models.
Initial Reception and Impact of Bohr’s Atomic Model
When Niels Bohr first introduced his atomic model in 1913, many in the scientific community were hesitant to accept it due to the groundbreaking concept of quantum jumps which appeared contradictory to the established principles of classical physics. Nevertheless, the notable accuracy of Bohr’s model in predicting the hydrogen atom’s spectrum could not be ignored and thus drew substantial interest.
Despite having its share of imperfections, the Bohr model importantly bridged the gap between Rutherford’s model and the future, more precise quantum mechanical models. It played an essential role in guiding subsequent explorations into atomic structure and eventually paved the way for the advent of quantum mechanics.

Bohr’s significant contribution to science was duly acknowledged when he was bestowed with the Nobel Prize in Physics in 1922, specifically for his efforts in probing the structure of atoms and the radiation they emit. Regardless of the emergence of more sophisticated atomic models over the years, the Bohr model remains an imperative teaching tool in both the fields of chemistry and physics.
2. The Basics of Bohr Atomic Model
Foundational Principles of Bohr’s Atomic Model
Niels Bohr’s Atomic Model, often referred to as the Bohr Model, is essentially a planetary system in miniature. Proposed over a century ago in 1913, it offers a simplified explanation of atomic structure where electrons encircle the nucleus at fixed distances, akin to planets orbiting the sun.
Bohr Atomic Model and Energy Levels
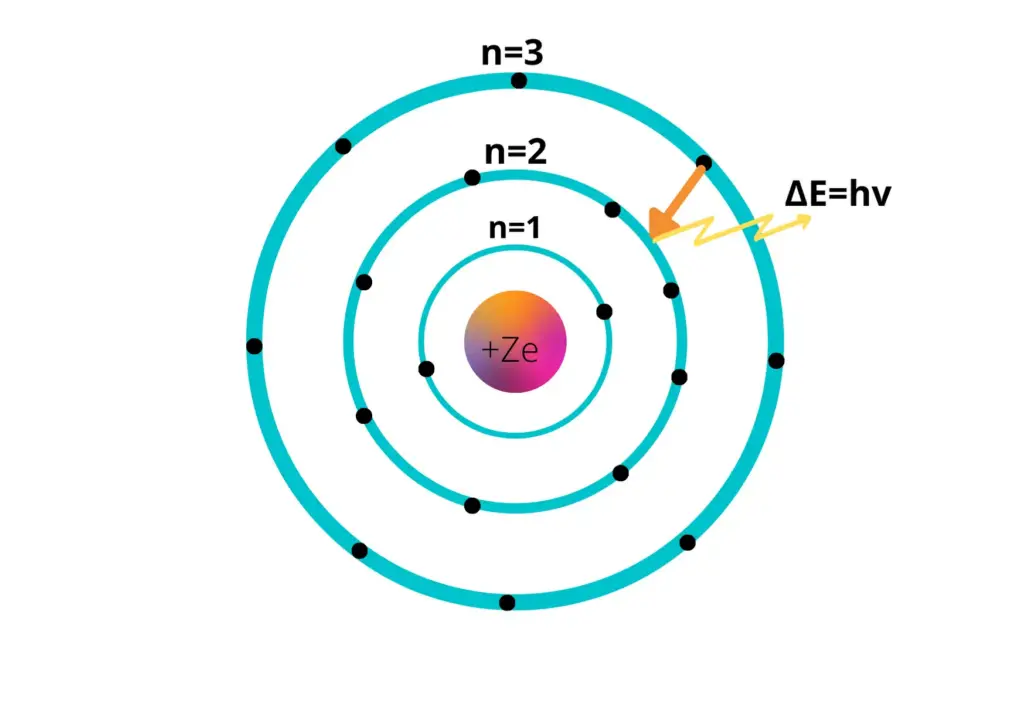
Within the Bohr Model, energy levels are referred to as electron shells and are represented by the principal quantum number (n). Bohr identified the energy levels using whole numbers starting from 1, 2, 3, and so on. The higher the number, the farther the energy level is from the nucleus, and therefore, the more energy the electron in that level possesses.
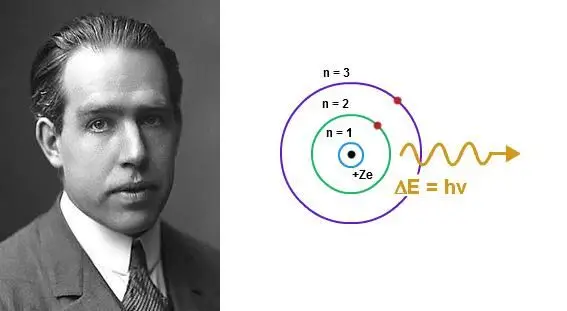
In other words, an electron that is far away from the nucleus resides in a high energy level and is, thus, high energy. Meanwhile, an electron close to the nucleus has low energy and resides at a low energy level. The energy level of an electron can change under the absorption or emission of energy, such as light.
Quantum Numbers in Bohr Model
In addition to the principal quantum number (n), which indicates the atomic energy level, the Bohr Model also introduces other quantum numbers. These are the angular momentum quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). Each one provides specific information about the electron’s orbitals‘ properties, such as shape, orientation, and spin characteristics.
Read Also
- Matter | Nature of matter | classification of matter, class 11 | some basic concepts of chemistry
- Physical and chemical properties of matter chemistry class 11
- Atomic mass and molecular mass: chemistry class 11, NCERT
The Postulates of Bohr Model
- Electrons revolve around the nucleus of an atom in fixed orbits without the emission of radiant energy.
- Electrons emit radiation when they jump from a higher energy level to a lower energy level. The wavelength (or frequency) of the emitted radiation is directly related to the energy difference between the two levels.
- The angular momentum of an electron in an orbit is an integral multiple of h/2π, where h is Planck’s constant.
These postulates were groundbreaking at the time and formed the foundation of what later became quantum mechanics.
Characteristics of Bohr’s Atomic Model
Notable for its simplicity and easy visualization, the Bohr model offers a clear, organized illustration of the atom’s structure. Its major success was the accurate prediction of the hydrogen spectrum, specifically the Balmer series. This series of spectral lines, previously unaccounted for, found an explanation in Bohr’s model.
However, it’s important to note that the model falls short when describing complex atomic behaviors, particularly for systems more sophisticated than a single-electron system like hydrogen. The encounter of these limitations spurred the development of more progressive models, like the Quantum Mechanical Model of the atom.
3. The Success and Limitations of Bohr Atomic Model
Success of the Bohr Atomic Model
Introduced by Niels Bohr in 1913, the Bohr model, often referred to as the Rutherford-Bohr model, was one of the first successful quantum mechanical descriptions of the atom. The model elevated the existing atomic understanding of the time, significantly building on previous models developed by Rutherford and other scientists. Its success largely resides in its simplicity and explanatory capabilities, with the conspicuous validation of its predictions coming from the spectrum of light emitted by hydrogen.
Looking from a more expansive perspective, the Bohr model played a critical role in the emergence of quantum mechanics. Bohr’s vision of the atom, composed of a positively charged nucleus surrounded by electrons in fixed orbits, aligned with the emerging quantum theory, opening the gates to the concepts of energy levels and quantum leaps, concepts that are foundational in quantum theory.
Hydrogen Spectral Lines and the Bohr Model
The Bohr model’s most noteworthy success is its explanation for the Balmer series of hydrogen spectral lines. The Balmer series refers to the visible spectral lines of the hydrogen atom. Using the Bohr model, the emission spectra of hydrogen could be calculated with remarkable accuracy.
In Bohr’s model, when an electron moves from a higher energy level to a lower one, it emits energy in the form of light. The energy of this light corresponds to a specific spectral line. Conversely, when an electron absorbs energy, it moves to a higher orbit, and this energy corresponds to a specific absorption spectrum.
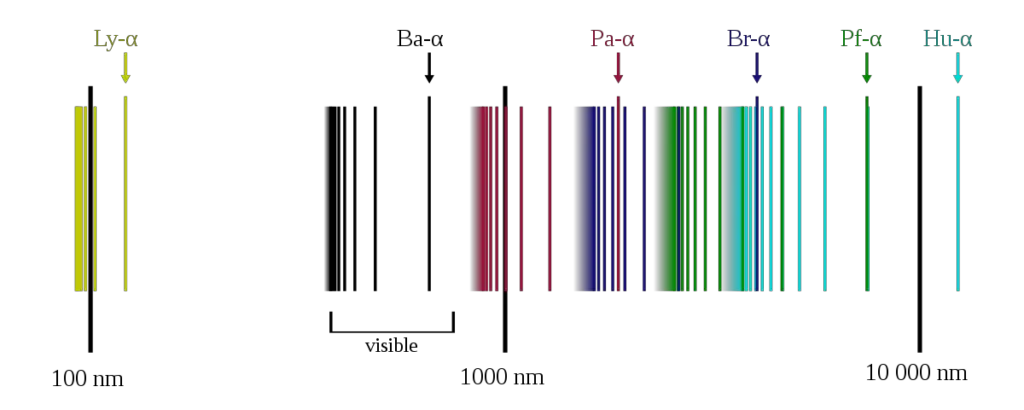
Bohr’s model was able to determine the frequency of the light being emitted or absorbed. Using the Rydberg formula, adapted to fit the Bohr model, all observable spectral lines of hydrogen could be accurately calculated. This was considered a tremendous accomplishment at the time and affirmed the success of Bohr’s model.
Limitations of the Bohr Atomic Model
Despite its success, the Bohr atomic model has a number of limitations. Firstly, it only accurately describes the energy levels of hydrogen, the simplest atom. For multi-electron atoms, it does not accurately predict their spectral lines or their chemical behavior.
Secondly, it treats electrons as particles orbiting around the nucleus in the way planets orbit the sun. But, quantum mechanics suggests that electrons do not behave like conventional particles. They exhibit wave-particle duality, meaning they can behave as both particles and waves.
Thirdly, the model implies that electrons in different orbits shouldn’t affect each other, but that’s not accurate. In reality, electrons interact with each other, and those interactions influence their energy levels.
Lastly, the Bohr model falls short when subjected to rigorous experimental tests. It fails to accurately predict the outcome of the Stern-Gerlach experiment, which demonstrated that electrons and other particles possess intrinsic angular momentum (spin).
Bohr’s Model: A Milestone in Atomic Theory
Despite its eventual supplanting by the quantum mechanical model, the Bohr model remains a cornerstone in the field of physics and chemistry for both its historic contribution and its educational importance.
Even with its known limitations, the Bohr model serves as an essential stepping stone to understanding the intricate world of quantum mechanics. It also played a pivotal role in the development of groundbreaking theories and models which shaped our present comprehension of atomic structure and quantum physics.
Read Also:
- Rutherford atomic model: postulates, observations, and limitations, class 11
- Thomson model of atom: postulates, drawbacks, & significance, class 11
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Discovery of Electron class 11: chemistry, NCERT
- Discovery of proton class 11: chemistry NCERT
4. Transition from Bohr Model to Quantum Mechanics
Navigating the Transition: From Bohr Model to Quantum Mechanics
Niels Bohr’s atom model, presented in 1913, marked a major milestone in atomic physics. Bohr introduced a model likening electrons to planets orbiting a nucleus, much like planets in our solar system orbit the sun. This model successfully detailed the atomic spectrum of hydrogen and reconciled physics with chemistry by explaining the structure of the periodic table.
Despite its revolutionary insights, the Bohr model fell short in its predictions for atoms with more than one electron. Additionally, it didn’t align well with the emerging principles of quantum physics. These inherent shortcomings largely triggered the scientific community’s transition to the era of Quantum Mechanics.
Wave-Particle Duality and Uncertainty Principle
The fundamental concept that challenged Bohr’s model was the wave-particle duality principle proposed by Louis de Broglie in 1926. According to this principle, particles such as electrons exhibit properties of both waves and particles. This contradicted Bohr’s model as it assumed the electron to be a particle orbiting the nucleus.
Following this, Werner Heisenberg’s Uncertainty Principle further shook the foundations of Bohr’s model. The uncertainty principle proposed that it is inherently impossible to simultaneously measure an electron particle’s position and momentum to full precision. This was against the Bohr model’s notion of predetermined orbits for electrons.
Schrödinger and Development of Quantum Mechanics
Then came Erwin Schrödinger, who made a significant breakthrough in the shift from the Bohr model towards quantum mechanics by developing the Schrödinger Equation in 1926. It described the behavior of quantum particles through wave functions rather than precise trajectories. This meant that instead of talking about electrons as particles moving in well-defined orbits, they became ‘clouds’ or ‘orbitals’ with a probability distribution around the nucleus.
Concept of Quantum Numbers and Atomic Orbitals
$\Psi$, the wave function in Schrodinger’s equation, could be defined by three ‘quantum numbers’. This led to the concept of atomic orbitals where each orbital in an atom is uniquely identified by three quantum numbers. Later, the concept of a fourth quantum number known as the ‘spin quantum number’ was introduced by George Uhlenbeck and Samuel Goudsmit.
Matrix Mechanics
Around the same time as Schrödinger, Werner Heisenberg developed his matrix mechanics, a new and abstract approach that completely discarded the idea of classical trajectories. He posited that the properties of particles were represented as matrices, and their behavior could be tracked using mathematical operations on these matrices.
The Great Shift in the Study of Atoms
By the end of the 1920s, a significant paradigm shift had occurred in the scientific world. Quantum mechanics had replaced the traditional understanding of atomic structures once provided by Bohr’s atomic model. This noteworthy departure veered away from the deterministic perspective offered by the classical model, introducing instead a probabilistic interpretation of the atomic environment.
The true significance of this quantum model lay in its unparalleled ability to accurately predict electron behaviors within atoms. Consequently, a thorough understanding of atomic structure was attained, effectively transforming the field of atomic physics for the better.
5. The Contemporary Understandings and Applications of the Bohr Atomic Model
Modern Applications and Perceptions of the Bohr Atomic Model
Over time, the Bohr atomic model has undergone considerable changes in interpretation and understanding since its original conceptualization in 1913. In the present day, this model is recognized chiefly for its utility in early physics education, offering students a basic comprehension of atomic structures. Many high school and undergraduate programs utilize Bohr’s model, due to its easily digestible yet illuminating graphical depiction of atomic components.
Bohr’s atomic model describes the atom as a central nucleus surrounded by orbiting electrons, each within distinct energy levels or ‘shells’. Furthermore, according to this model, electrons can transit between these energy levels by absorbing or emitting discrete bundles of energy, or ‘quanta’. This unique aspect of Bohr’s model sheds light on previously incomprehensible aspects of atomic structures, such as the stability of atoms and the emission spectra of hydrogen, as explained by traditional physics.
As our understanding of atomic structures continues to evolve, the quantum mechanical models of the atom in use today still uphold the quantization of energy, a key concept introduced by Bohr.
Read Also:
- Difference between isobars and isotopes (Tabular form)
- Isobars and Isotopes: definition, examples, and differences, class 11
- Atomic number and atomic mass class 11
- Rutherford gold foil experiment class 11
Applications of Bohr Atomic Model in Modern Science
Even with its simplifications and certain inaccuracies, Bohr’s model still has pertinent applications in contemporary science and technology.
For instance, the model helps in the analysis of atomic spectra. When an atom absorbs energy, its electrons get excited and subsequently emit energy as they return to their original state. This emission appears as a spectral line, and Bohr’s model enables the calculation of these lines, especially for a simple, single-electron atom like hydrogen.
Further, Bohr’s model is often used in introducing concepts of quantum physics. Quantum physics, which is crucial to the development of modern technology like semiconductor electronics and quantum computers, relies on Bohr’s fundamental idea of energy in ‘packets’ or quanta.
Beyond physics, Bohr’s atomic model also finds application in the field of chemistry. It helps in explaining the periodic table— the arrangement of elements according to their atomic numbers and electronic configuration. It also fosters an understanding of chemical bonding and the formation of molecules.
Despite the advent of more complex and accurate atomic models, Bohr’s atomic model still holds its ground for its simplicity and ability to fundamentally explain atomic behavior. It serves as a key stepping stone in atomic theory, aiding physicists, chemists, and students in understanding the intriguing world of atoms.
As we reflect upon the remarkable journey of Bohr’s Atomic Model, it’s abundantly clear that its influence reverberates throughout the realms of scientific discourse and discovery to this very day. Beyond its academic relevance, Bohr’s model holds practical implications for a myriad of technological applications, cementing its enduring relevance in modern-day science.
While its inherent limitations encouraged the development of Quantum Mechanics, the elementary principles of the Bohr Model continue to offer invaluable insights into the atomic structure. Ultimately, it is through these scientific shifts and evolutions that we truly appreciate the dynamism of scientific knowledge and the eternal quest for understanding the universe’s intricate machinations.
Stay tuned with Laws Of Nature for more useful and interesting content.
This article was effortlessly written by Writio.