Atomic number and atomic mass
Atoms, the tiny building blocks of everything around us, have two important properties: atomic number and atomic mass. These characteristics of atoms help us understand different elements and how they behave. Whether you’re a student studying chemistry or just curious about the world, knowing about atomic numbers and atomic mass will give you a better understanding of the atoms that make up everything.

Think about a world where everything is disorganized and doesn’t make sense. It would be hard to learn or understand anything. Thanks to the atomic number that comes and plays an important role in the organization, and helps to make sense. It gives each element a specific number that helps us organize them. From hydrogen to uranium, each element has its own unique atomic number. This number helps us figure out what an element is and tells us how it relates to other elements.
But wait, there’s more! Atomic mass is another important factor. It tells us how heavy an atom is by considering its protons, neutrons, and electrons. By understanding atomic mass, we can compare elements and learn about isotopes. Isotopes are versions of elements with different numbers of neutrons but the same atomic number. Understanding atomic mass helps us see the differences between elements and how they behave.
In this article, we’ll discuss what is atomic number and atomic mass, and its significance. So let’s join us on a journey into the world of atoms and discover the fascinating secrets of atomic number and atomic mass.
What is an Atomic number?
Atomic number definition: The atomic number of an element is the number of protons found in the nucleus of an atom of that element.
Atoms, the microscopic building blocks of matter, come in a large number of types, each with its own distinct properties. But how do we keep track of these countless elements? This is where the concept of atomic number steps in as a guiding light, providing us with a systematic way to identify and categorize elements.
In simple terms, an atomic number is like a unique ID card for each element. It tells us how many protons an atom of that element has in its nucleus. Protons are positively charged particles that reside in the center of an atom. By counting the number of protons, we can determine the atomic number, which remains constant for a specific element.
Imagine you have a mysterious atom. You examine it closely and find that it contains six protons. Voila! The atomic number of this atom is 6. Based on this information alone, we can confidently identify the atom as carbon, because carbon atoms always have six protons. Similarly, an atom with eight protons is always oxygen, and an atom with 79 protons is always gold.
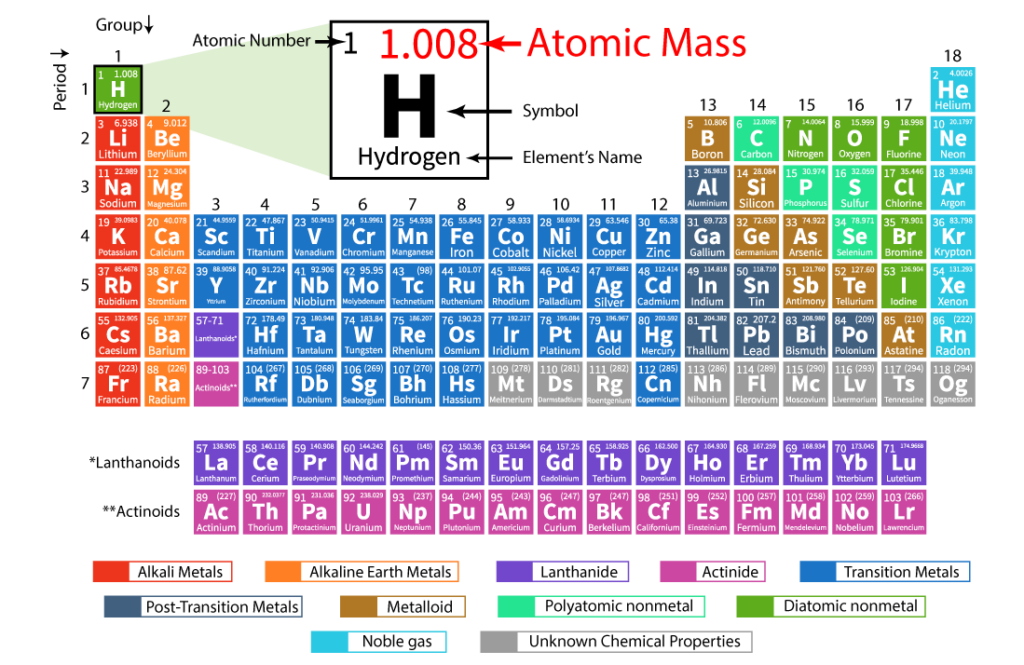
The atomic number not only gives us a way to identify elements but also reveals patterns and relationships among them. Elements are arranged in order of increasing atomic number in the periodic table, a tabular arrangement of elements that organizes them based on their properties. This organization allows us to easily locate and compare elements with similar characteristics.
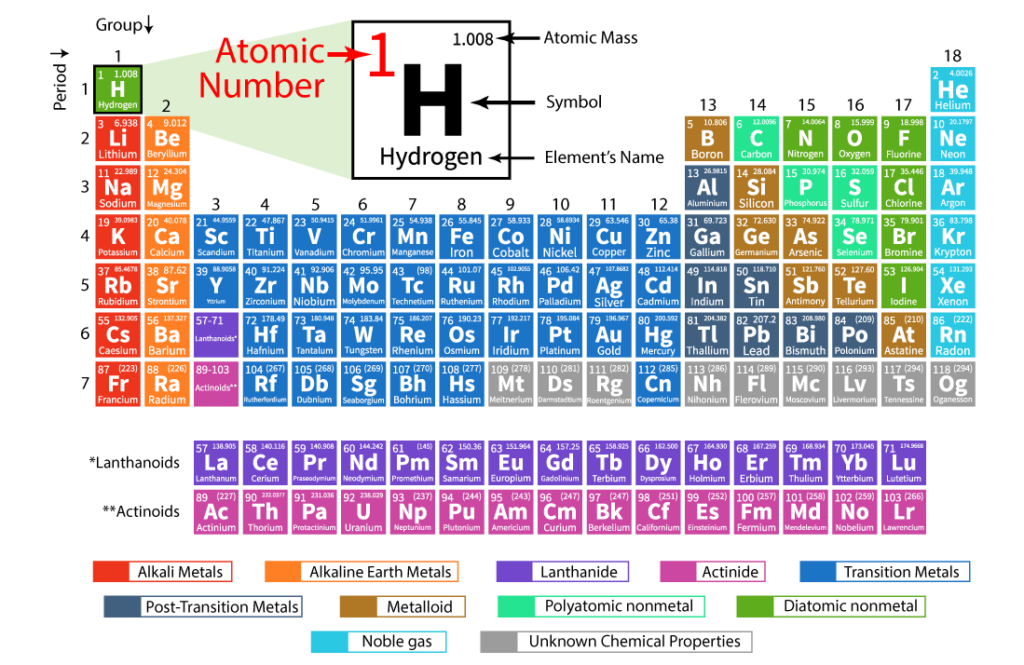
One interesting feature of the atomic number is that it determines an element’s place in the periodic table. Elements with low atomic numbers, such as hydrogen and helium, are found at the top left, while elements with higher atomic numbers, like uranium and plutonium, are situated towards the bottom right. This arrangement helps us visualize how elements change as we move across and down the periodic table. As you can see above.
Read Also:
- Discovery of Electron class 11: chemistry, NCERT
- Discovery of proton class 11: chemistry NCERT
- Discovery of Neutron class 11: history, properties, observations, and Conclusions
- Millikan oil drop experiment class 11: history, apparatus, procedure, observation
As we delve deeper into the atomic world, we encounter isotopes, which are different versions of the same element that have the same atomic number but different atomic masses. Isotopes occur when atoms have varying numbers of neutrons, which are neutral particles found in the nucleus. For example, hydrogen has three isotopes: hydrogen-1 (also called protium), hydrogen-2 (deuterium), and hydrogen-3 (tritium), all with one proton but differing numbers of neutrons.
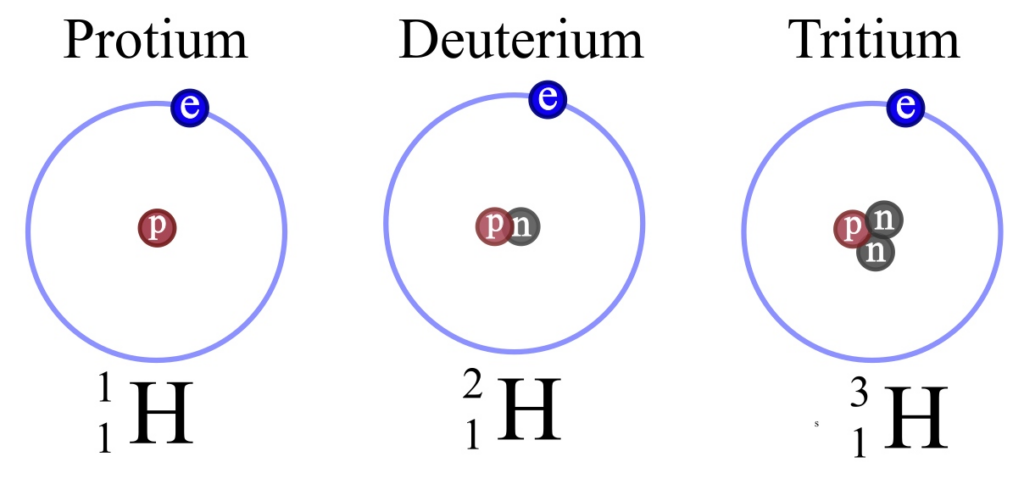
Isotope definition: An isotope is a variant of an element that has the same number of protons in its nucleus but a different number of neutrons.
Atomic number key points
- Atomic number is like a unique ID card for each element.
- It represents the number of protons in an atom’s nucleus.
- Atomic number helps identify and categorize elements.
- Elements are organized in the periodic table based on increasing atomic numbers.
- Low atomic number elements are found at the top left, while higher atomic number elements are towards the bottom right.
- Isotopes are different versions of an element with the same atomic number but different numbers of neutrons.
- Isotopes occur due to varying numbers of neutrons in the nucleus.
- Understanding atomic numbers is essential for studying chemistry and identifying elements accurately.
What is Atomic mass?
Atomic mass definition: Atomic mass is the measure of the mass of an atom. It represents the combined mass of protons, neutrons, and electrons in the atom.
Atoms, the tiny building blocks of matter, have not only unique identities but also varying weights. This weight is captured by the concept of atomic mass, which helps us understand the heaviness of individual atoms and their role in the composition of elements.
In simple terms, atomic mass refers to the total mass of an atom. It takes into account the combined masses of protons, neutrons, and electrons that make up an atom. Protons and neutrons reside in the atom’s nucleus and are collectively known as nucleons, while electrons orbit around the nucleus.
To determine the atomic mass of an atom, we add up the masses of protons and neutrons. Both protons and neutrons have a mass of approximately 1 atomic mass unit (amu). Electrons, on the other hand, have a much smaller mass, so they contribute very little to the overall atomic mass.
For example, let’s consider carbon. A carbon atom typically has 6 protons and 6 neutrons. Adding their masses together gives us a total mass of 12 amu for a carbon atom. The number of electrons, which is also 6 for carbon, does not significantly impact the atomic mass due to their negligible mass.
It’s important to note that atomic mass is not a whole number for most elements. This is because most elements have multiple isotopes, each with a different number of neutrons. Isotopes of an element have the same atomic number (number of protons) but different atomic masses (due to different numbers of neutrons). The atomic mass listed on the periodic table represents the average mass of all the naturally occurring isotopes of an element, taking into account their relative abundance.
Atomic mass key points
- Atomic mass refers to the total mass of an atom.
- It includes the combined masses of protons and neutrons in the nucleus.
- Electrons contribute very little to the overall atomic mass.
- Atomic mass is determined by adding the masses of protons and neutrons.
- Most elements have multiple isotopes, resulting in non-whole number atomic masses.
- Atomic mass helps compare elements based on relative weights.
- It is used in stoichiometry calculations for chemical reactions.
- Atomic mass is important in nuclear chemistry and the study of radioactive isotopes.
- Understanding atomic mass provides insights into the composition and behavior of elements.
Read Also:
- Rutherford atomic model: postulates, observations, and limitations, class 11
- Thomson model of atom: postulates, drawbacks, & significance, class 11
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Rutherford gold foil experiment class 11
Watch this video for more reference.
Difference Between Atomic Number and Atomic Mass
Key differences between atomic number and atomic mass are given below:
Atomic Number:
- Atomic number represents the number of protons in an atom’s nucleus.
- It is a unique identifier for each element.
- Atomic number determines the element’s identity and its position in the periodic table.
- It remains constant for a specific element.
- Elements with the same atomic number have similar chemical properties.
- Atomic number is denoted by the symbol “Z.”
Atomic Mass:
- Atomic mass refers to the total mass of an atom.
- It includes the combined masses of protons, neutrons, and electrons in an atom.
- Atomic mass is not a whole number for most elements due to the presence of isotopes.
- Isotopes have the same atomic number but different atomic masses.
- Atomic mass is listed on the periodic table as the average mass of all naturally occurring isotopes.
- Atomic mass is denoted by the symbol “A” or represented in atomic mass units (amu).
In summary, the key differences between atomic number and atomic mass are:
- Atomic number is the number of protons and defines an element’s identity, while atomic mass is the total mass of an atom.
- Atomic number remains constant for an element, while atomic mass varies depending on the isotopes present.
- Atomic number determines an element’s position in the periodic table, while atomic mass helps compare the relative weights of different elements.
- Atomic number is denoted by “Z,” and atomic mass is denoted by “A” or measured in atomic mass units (amu).
Significance of atomic number and atomic mass
The significance of atomic number and atomic mass lies in their roles in understanding the properties and characteristics of elements. Here’s a closer look at the significance of each:
Atomic Number:
- Element Identification: Atomic number serves as a unique identifier for each element. It distinguishes one element from another, allowing scientists to identify and categorize elements accurately.
- Periodic Table Organization: The atomic number determines the position of an element in the periodic table. Elements are arranged in order of increasing atomic number, which reveals patterns, trends, and relationships among elements. It helps in understanding the similarities and differences between elements and their properties.
- Chemical Behavior: Elements with the same atomic number have the same number of protons and thus share similar chemical properties. The atomic number provides insight into an element’s reactivity, bonding behavior, and participation in chemical reactions.
Atomic Mass:
- Isotope Differentiation: Atomic mass takes into account the presence of isotopes, which are different versions of an element with the same atomic number but different atomic masses due to varying numbers of neutrons. Atomic mass helps distinguish between isotopes and understand their relative abundance in nature.
- Stoichiometry and Chemical Calculations: Atomic mass is used in stoichiometry, where it enables the calculation of the quantities of substances involved in chemical reactions. It aids in determining the amount of reactants needed, predicting product yields, and performing various chemical calculations.
- Nuclear Chemistry and Radioactivity: Atomic mass is crucial in nuclear chemistry, as it helps in the study of radioactive isotopes and their decay. It provides information about the stability and half-life of isotopes, which is essential for applications such as radiometric dating, medical imaging, and nuclear energy production.
Atomic number and atomic mass 1 – 30
Here is the atomic number and atomic mass of the element from 1 to 30.
| Atomic Number | Element Symbol | Atomic Mass |
|---|---|---|
| 1 | H | 1.008 |
| 2 | He | 4.0026 |
| 3 | Li | 6.94 |
| 4 | Be | 9.0122 |
| 5 | B | 10.81 |
| 6 | C | 12.01 |
| 7 | N | 14.01 |
| 8 | O | 16.00 |
| 9 | F | 19.00 |
| 10 | Ne | 20.18 |
| 11 | Na | 22.99 |
| 12 | Mg | 24.31 |
| 13 | Al | 26.98 |
| 14 | Si | 28.09 |
| 15 | P | 30.97 |
| 16 | S | 32.07 |
| 17 | Cl | 35.45 |
| 18 | Ar | 39.95 |
| 19 | K | 39.10 |
| 20 | Ca | 40.08 |
| 21 | Sc | 44.96 |
| 22 | Ti | 47.87 |
| 23 | V | 50.94 |
| 24 | Cr | 52.00 |
| 25 | Mn | 54.94 |
| 26 | Fe | 55.85 |
| 27 | Co | 58.93 |
| 28 | Ni | 58.69 |
| 29 | Cu | 63.55 |
| 30 | Zn | 65.38 |
Chronology of the history of atomic number
The history of atomic numbers is closely intertwined with the development of our understanding of atoms and their composition. Below is a brief chronology of the history of the atomic numbers:
- Early Atomic Theories:
- In the 5th century BCE, ancient Greek philosophers such as Leucippus and Democritus proposed the concept of atoms as indivisible particles that make up all matter.
- However, it was not until the early 19th century that experimental evidence began to support the atomic theory proposed by John Dalton, who suggested that elements are made up of atoms with unique characteristics.
- Discovery of Atomic Weight:
- In the early 19th century, chemists such as John Dalton, Jöns Jacob Berzelius, and others started investigating the relative weights of atoms and compounds.
- Dalton proposed the concept of atomic weights, representing the ratios of elements in chemical reactions. This laid the foundation for understanding the composition of compounds.
- Law of Triads and Octaves:
- In the mid-19th century, chemists such as Johann Wolfgang Döbereiner and John Newlands observed patterns among elements.
- Döbereiner proposed the Law of Triads, which highlighted the existence of groups of three elements with similar properties, where the atomic weight of the middle element was roughly the average of the other two.
- Newlands further expanded on this idea and introduced the Law of Octaves, which observed a repeating pattern for every eighth element when ordered by increasing atomic weight.
- Mendeleev’s Periodic Table:
- In 1869, Dmitri Mendeleev, a Russian chemist, published his groundbreaking periodic table.
- Mendeleev organized the elements based on their increasing atomic weights and observed periodic patterns in their properties.
- He left gaps in the table for undiscovered elements and predicted their properties based on the trends observed in the existing elements.
- Discovery of Atomic Number:
- In the early 20th century, scientists such as Henry Moseley, working independently, made significant breakthroughs in understanding atomic structure.
- Moseley conducted X-ray experiments on various elements and found a relationship between the X-ray spectra and the atomic number of elements.
- Moseley proposed that the atomic number, representing the number of protons in the nucleus, should be the organizing principle of the periodic table, rather than atomic weight.
- Acceptance of Atomic Number:
- By the 1920s, the concept of atomic number gained acceptance in the scientific community.
- The periodic table was reorganized based on atomic number, as proposed by Moseley, which led to a more accurate understanding of element properties and trends.
- The discovery of isotopes and the realization that atomic number determines an element’s identity further solidified the importance of atomic number in modern atomic theory.
Today, the atomic number remains a fundamental concept in chemistry and serves as a key identifier for elements in the periodic table, reflecting our understanding of the fundamental nature of atoms and their arrangement in the universe.
Read Also:
- John dalton’s atomic theory: postulates & limitations, class 11, NCERT
- Uncertainty in measurement chemistry, class 11 NCERT
- Laws of chemical combination: chemistry class 11, NCERT
- Importance of chemistry in our everyday life, class 11
Notation of Atom
The notation of an atom represents its symbol, atomic number, and atomic mass. The position symbol, atomic number, and atomic mass is defined below:
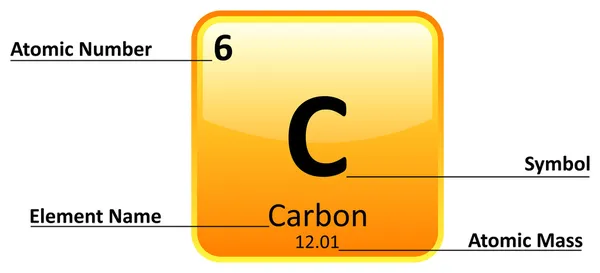
- Symbol: The symbol represents the element and is typically derived from its name. For example, “H” represents hydrogen, “O” represents oxygen, and “Fe” represents iron.
- Atomic Number: The atomic number is denoted by the symbol “Z” and represents the number of protons in an atom’s nucleus. It determines the element’s identity and its position in the periodic table. The atomic number is typically written as a subscript to the left top of the element symbol. For example, “6C” represents the element carbon, where “6” is the atomic number.
- Atomic Mass: The atomic mass is denoted by the symbol “A” or sometimes written as a superscript to the right top or bottom of the element symbol. It represents the total mass of an atom, including the combined masses of protons and neutrons. Atomic mass is often listed as a decimal number since it accounts for the existence of isotopes and their relative abundances. For example, “12.01C” represents carbon with an atomic mass of approximately 12.01 atomic mass units.
Frequently Asked Questions – FAQs
What is an Atomic number?
The atomic number of an element is the number of protons found in the nucleus of an atom of that element.
What is Atomic mass?
Atomic mass is the measure of the mass of an atom. It represents the combined mass of protons, neutrons, and electrons in the atom.
What is the significance of the atomic number in determining an element’s identity?
The atomic number represents the number of protons in an atom’s nucleus, which is unique to each element. It serves as the fundamental identifier of an element, allowing scientists to differentiate between different elements based on their atomic number.
How does atomic mass differ from atomic number?
Atomic mass represents the total mass of an atom, taking into account the combined masses of protons, neutrons, and electrons. It can vary due to the presence of isotopes, whereas the atomic number is the number of protons in the atomic nucleus.
What is the difference between atomic mass and molar mass?
Atomic mass refers to the mass of a single atom, while molar mass is the mass of one mole of a substance, which is calculated by summing up the atomic masses of its constituent atoms. Molar mass is expressed in grams per mole (g/mol) and is used in various chemical calculations.
Why is it important to understand the concept of isotopes in relation to atomic mass?
Isotopes are different versions of an element with the same atomic number but different atomic masses due to varying numbers of neutrons. Understanding isotopes is crucial because they can have different physical and chemical properties, affect the overall atomic mass of an element, and play a role in various scientific applications such as radiometric dating, medical imaging, and nuclear energy production.