The Rutherford model of the atom was proposed by Ernest Rutherford in 1911 and was a major breakthrough in understanding the structure of the atom. It replaced the earlier Thomson model of an atom (plum pudding model) and provide a new way of understanding the structure of the atom.
However, despite its significant contributions to the understanding of atomic structure, the Rutherford model has certain drawbacks that limit its accuracy and applicability. In this article, we will discuss the drawbacks of the Rutherford model of atom and understand why it was eventually replaced by newer, more accurate models. So let’s get started…
Rutherford’s gold foil experiment class 11
Ernest Rutherford was curious about the nature of the positive charge in atoms and wanted to test whether the Thomson model of an atom accurately described the structure of the atom or not. To test the prevailing model of the atom at the time that is the Thomson model. He performed the gold foil experiment to study the structure of atoms.
The Thomson model suggested that atoms were a homogeneous sphere of positive charge with electrons embedded in them.
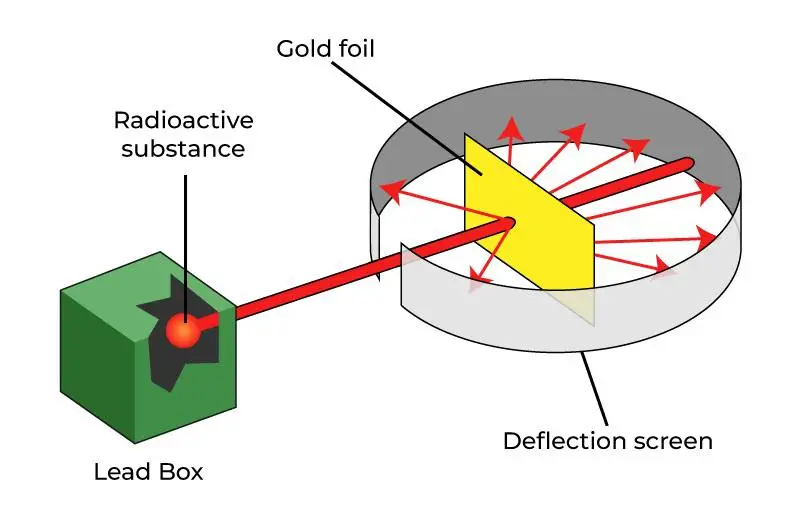
To perform the experiment, he choose to use gold foil for his experiment because it was a thin and malleable material that could be made into very thin sheets. Rutherford directed a beam of alpha particles at the gold foil and observed the behavior of the particles as they passed through the foil.
The observations of the experiment led to the development of a new model of the atom, called the Rutherford atomic model, which described the atom as a small, dense nucleus surrounded by electrons in circular orbits.
Read Also:
- Difference between isobars and isotopes (Tabular form)
- Isobars and Isotopes: definition, examples, and differences, class 11
- Atomic number and atomic mass class 11
- Rutherford gold foil experiment class 11
Here are some important observations made by Rutherford from the gold foil experiment.
- Most of the alpha particles passed straight through the gold foil.
- A small fraction of alpha particles were deflected at large angles.
- Some alpha particles bounced back toward the source.
- The deflection and bouncing back of the alpha particles suggested that there was a tiny, positively charged nucleus at the center of the atom.
- The nucleus was relatively large and dense compared to the size of the alpha particles.
- The deflection of the alpha particles was due to the repulsive electrostatic forces between the positively charged alpha particles and the positively charged nucleus.
- The fact that most of the alpha particles passed straight through the foil suggested that the atom was mostly empty space.
- These observations led to the development of the Rutherford model of the atom, which described the atom as a small, dense nucleus surrounded by electrons that orbit in circular paths.
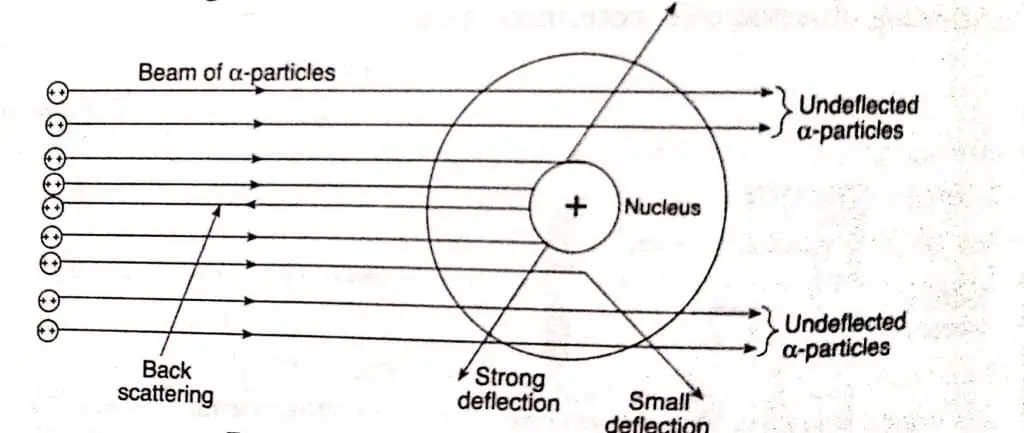
Rutherford atomic model key features
On the basis of the observations made by him during the gold foil experiment. He proposes his atomic model, also known as the nuclear model. Here are the key features of the Rutherford atomic model:
- The atom is made up of a small, dense, positively charged nucleus located at the center of the atom.
- The nucleus contains most of the mass of the atom but occupies a very small volume compared to the overall size of the atom.
- Electrons orbit the nucleus in circular paths, similar to the way planets orbit the sun.
- The electrons are negatively charged and are held in their orbits by the electrostatic attraction between the negatively charged electrons and the positively charged nucleus.
- The number of electrons in an atom is equal to the number of protons in the nucleus, resulting in a neutral overall charge.
- The Rutherford model of the atom laid the foundation for the development of the modern quantum mechanical model of the atom, which describes the behavior of electrons in more detail.
Drawbacks of Rutherford’s model of atom
The Rutherford model proposed that atoms had a small, positively charged nucleus at the center, surrounded by negatively charged electrons orbiting around it. This model explained the results of the gold foil experiment, but it had some drawbacks. Here are some drawbacks of Rutherford’s model of atom:
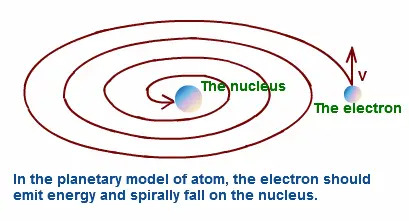
- Stability of the atom: According to the laws of electromagnetism, accelerated charges should emit energy in the form of electromagnetic radiation. Therefore, the orbiting electrons in the Rutherford model would lose energy and spiral into the nucleus, causing the atom to collapse. This contradicted the stability of the atom, which is observed in nature.
- Spectral lines: The Rutherford model could not explain the observed spectral lines of different elements. Each element emits or absorbs radiation at specific wavelengths, creating a unique spectral line pattern. However, the Rutherford model could not explain why the electrons in an atom emitted or absorbed radiation only at certain discrete wavelengths.
- Quantization of angular momentum: The Rutherford model could not explain why the angular momentum of the electrons in an atom was quantized, meaning that it could only have certain specific values. This was observed in experiments, but it could not be explained by the Rutherford model.
To overcome these drawbacks, Niels Bohr proposed a modified atom model in 1913, which incorporated the idea of quantization of energy. The Bohr model of the atom explained the stability of the atom, the spectral lines, and the quantization of angular momentum, and it became the basis for modern atomic theory.
Read Also:
- Rutherford atomic model: postulates, observations, and limitations, class 11
- Thomson model of atom: postulates, drawbacks, & significance, class 11
- Cathode Tube Ray Experiment class 11: working, procedure, observation, and conclusion
- Discovery of Electron class 11: chemistry, NCERT
- Discovery of proton class 11: chemistry NCERT
Frequently Asked Questions – FAQs
What are the three drawbacks of the Rutherford model?
The three drawbacks of the Rutherford model are:
1). Couldn’t explain the stability of atoms.
2). Couldn’t explain atomic spectral lines.
3). Couldn’t explain the Quantization of angular momentum.
What is the Rutherford atomic model?
The Rutherford atomic model described that atoms had a small, positively charged nucleus at the center, surrounded by negatively charged electrons orbiting around it. This model is explained on the basis of the results of the gold foil experiment performed in 1911.
What were the observations of the Rutherford atomic model?
Rutherford’s main observations from the gold foil experiment are:
1). Most of the alpha particles passed straight through the foil, indicating that atoms are mostly empty space.
2). A small fraction of alpha particles were deflected by small angles, showing that there is a concentrated positive charge within the atom, leading to the discovery of the nucleus.
3). Very few alpha particles bounced back, further supporting the idea of a dense, positively charged central nucleus, which repels the positively charged alpha particles.
Why was Rutherford’s model unstable?
Rutherford’s model was unstable because it could not explain why the negatively charged electrons did not fall into the positively charged nucleus, leading to the collapse of the atom. This was known as the “radiation problem.” The problem was later resolved by Niels Bohr’s atomic model, which introduced the concept of quantized energy levels for electrons, providing a stable structure for the atom.
Why did Rutherford use gold foil?
Rutherford used gold foil in his famous experiment in order to study the properties of alpha particles and the structure of atoms. Gold was chosen because it is a malleable metal that could be hammered into very thin sheets (foils) of a few atoms thick.
When alpha particles were directed at the gold foil, Rutherford observed that some of the particles were deflected at large angles, indicating that the atoms in the gold foil had a small, dense, positively charged nucleus at their center. This experiment provided evidence for the existence of atomic nuclei and helped to establish the modern understanding of atomic structure.
Stay tuned with Laws Of Nature for more useful and interesting content.