When we talk about chemistry, then it means that we are talking about a branch of science that mainly deals with the composition, properties, and interaction of matters.
The matter is that thing, which chemistry studies. So it is very important for us that we understood the term matter and knew its basic characteristics nature. In this little piece of article, we are going to discuss the nature of matter. So let’s get started…
What is matter?
First of all, let’s discuss the term matter. So, what is the matter? Are your smartphone is a matter? Are your T.V, fan, sofa, clothes, water bottle, plastic, metals, etc. are all matters? Yes, absolutely! all these are matters, and even our body is also a matter. In fact, everything, you see with your naked eyes or by any means is matters, Why? It is because;
Anything which have mass and occupies space are called matter.
So everything you see in your surroundings is matters because they have mass and occupy space.
States of matters
So anything which has mass and occupies space is called matter. In this universe, we don’t know how many matters exist? Let leave the universe, even we didn’t know all the matters which present on the earth.

There are a large variety of matters present on this physical earth. If we want to study them then how can we do so? To ease in their study, broadly we classify the matters into three physical states on the basis of their molecular separation distance viz. solid, liquid and gases. In some text plasma and bose-einstein condensate are mentioned as the fourth and fifth states of matter. We ignore these two additional states of the matter here.
Solid
Solids are tough and rigid bodies. They have definite shape and volume. They have the least molecular separation distance. Some properties of solid-state are given below:
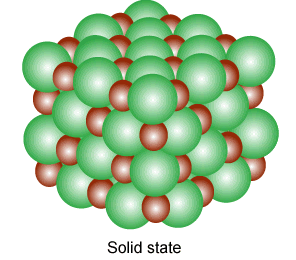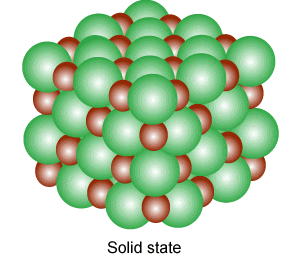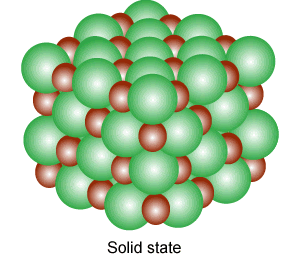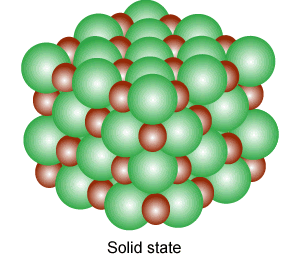
- They have strong intermolecular forces.
- They have low Kinetic energy.
- They have definite shape and volume.
- They are rigid and tough.
- They have least molecular seperation distance.
- They have comparatively higher density.
- They are incompressible.
- They have low diffusion rate (millions of times slower than liquids)
- It can be further categorised as crystalline and amorphous solids.
Liquids
They are not tough and rigid like solids but have definite volume but no definite shape. They have an intermediate molecular separation distance and can able to flow so they are also termed fluids. Liquids do have not a definite shape but they acquire the shape of that container in which they are kept. Some properties of liquids are given below:
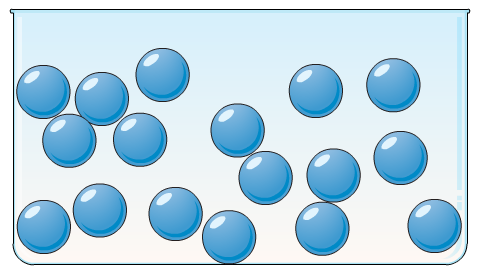
- They have moderately strong intermolecular forces.
- They are also called fluids because they can slip and slide over past each other.
- They have indefinite shape but definite volume.
- They are incompressible. You can’t lower it’s volume by compressing it.
- They have slightly less density than solid but more than gases.
- They have high diffusion rate than solids.
- They exhibit surface tension, capillary action and have viscosity.
Gas
This state of matter has no definite shape and volume. They have a large molecular separation distance due to which they have the weakest intermolecular forces. Molecules of the gas are free to move anywhere. They have also been termed fluids because of having flowing properties. They also acquire the shape of the container in which they are kept. Some properties of the gaseous state are given below:
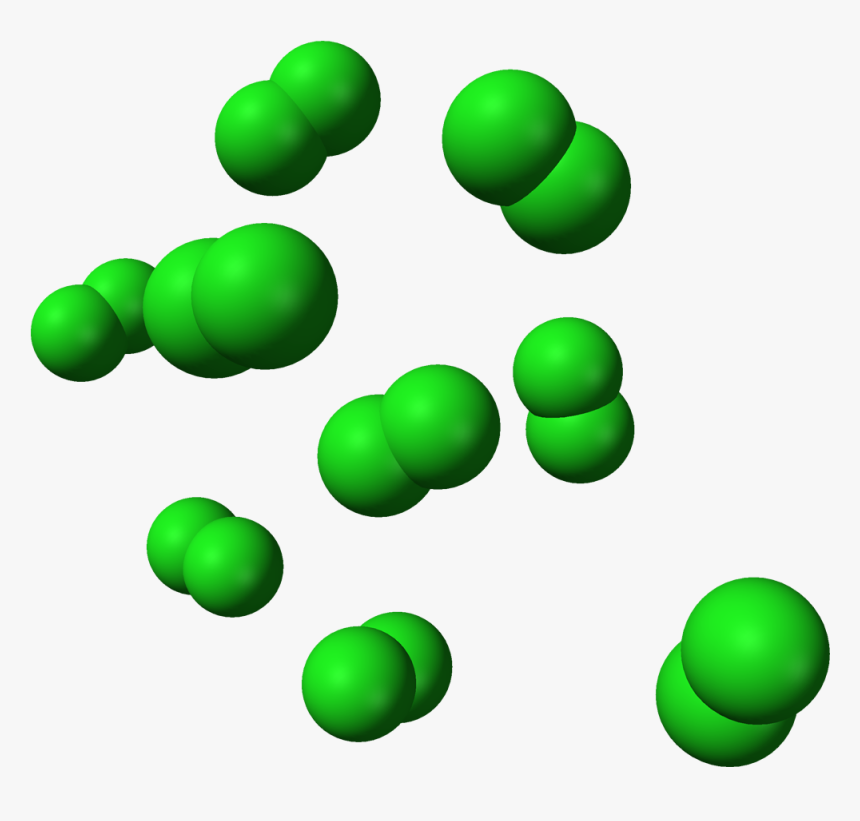
- Have not definite shape and volume.
- They have least intermolecular forces.
- And have Large molecular seperation distance.
- They are incompressible. You can bring closer the molecules of the gases by applying pressure and lower the temperature.
- They have high Kinetic energy.
- They have lowered density (approx. 1000 times less dense than the same substance as a liquid or solid).
- They have very high diffusion rate than solids and liquids.
- They exert pressure on the walls of the container in which they are kept.
- You can’t see this state of matter but you can feel it.
So, we have discussed the three states of matter and their properties. But the interesting thing between them is that these three states of matter are interconvertible by changing the conditions of pressure and temperature.
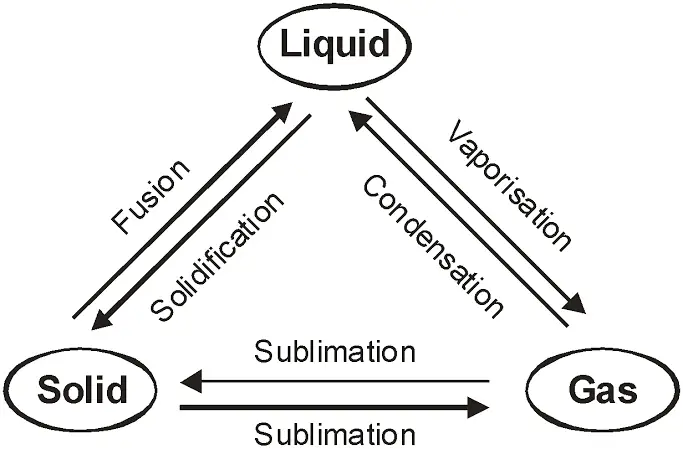
A solid can be converted into liquid if you supply heat to them and on further heating, a liquid can be changed into a gaseous or vapor state. But in the reverse process, a gas on cooling liquifies to the liquid, and the liquid on further cooling freezes to the solid.
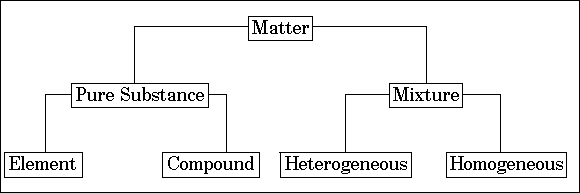
Classification of matter
At the macroscopic level, matter can be classified as a mixture and pure substance. See figure below:
Mixture
Many things present around you are mixtures. For example, sugar solutions in water, air, tea, etc. all are mixtures. So how can we define mixture?
A mixture is defined as – when two or more substances mix with each other without participating in a chemical change, then the resulting substance is called a Mixture.
In the mixture components, neither lose their individuality nor they combined chemically. A mixture is further subdivided into two categories that are the homogenous mixture and the heterogeneous mixture.
Homogenous mixture
In a homogenous mixture, components are completely mixed with each other and its composition is uniformly distributed throughout. Some examples of the homogeneous mixture are – sugar solutions, air, salt solution, etc.
Heterogenous mixture
In a heterogeneous mixture, the composition is not distributed uniformly throughout and sometimes different components can be observed. For example:- mixtures of salt and sugar, grain and pulses along with some dirt (often stone) pieces.

You can think of many more examples of heterogeneous as well as a homogenous mixture which you come across in daily life. It is important to note that components of the mixture can be separated by using physical methods such as simple hand-picking, filtration, crystallization, distillation, etc.
Pure substance
Pure substances have characteristics different from the mixtures. They have fixed composition, whereas mixtures may contain the components in any ratios and their composition may vary.
Pure substances are substances that are made up of only one kind of particles and has a fixed or constant composition.
Some examples of pure substances are copper, silver, gold, water, glucose, etc.
Characteristics and properties of pure substance
Some characteristics and properties of a pure substance are given below:
- It is almost homogenous in nature and contains only one types of particles.
- They have constant and uniform composition.
- They have fixed boiling and melting point.
- Pure substance usually participate in chemical reaction and give a predictable products.
A pure substance can be further subdivided into two categories such as elements and compounds.
Elements
An element is a pure substance that contains only one type of particle. These particles can be atoms or molecules. It cannot be broken down into a simpler type of matter by either physical or chemical means and can exist as either atom like argon or molecules nitrogen.
Sodium, copper, silver, nitrogen, hydrogen, etc. are some examples of elements. These all contain only one type of atom. But atoms of different elements are different in nature and properties.
When two or more atoms of same or different kinds of elements combined together chemically then it forms molecules.
When two or more atoms of the same elements are combined together chemically then it forms the molecules of the respective elements. For example, O2 is a molecule of oxygen that contains only oxygen atoms. Thus, it is a molecule of the element oxygen.
But when two or more atoms of different elements are combined together chemically then it forms the molecules of the compound. For example, when two atoms of hydrogen and one atom of oxygen are combined chemically then it forms one molecule of compound (water).
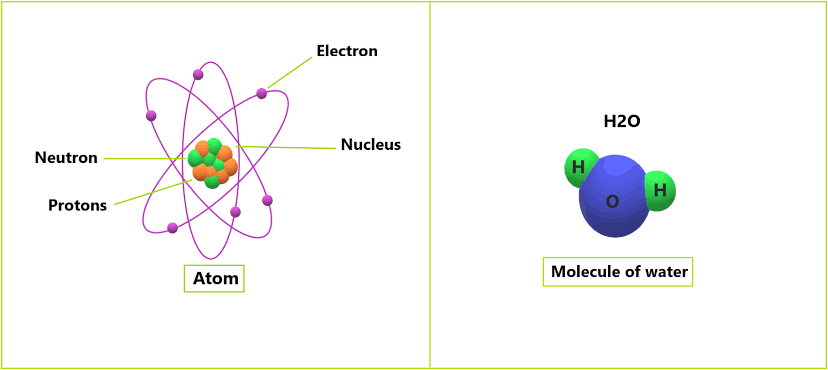
Compounds
When two or more atoms of different elements are combined together chemically then it forms the molecules of the compound. So to form compounds we need atoms of different elements.
A compound is nothing but a substance that results when two or more different elements combined in such a way that the atoms of the different elements are held together by chemical bonds that are difficult to break.
Examples of some compounds are water, ammonia, carbon dioxide, glucose, etc. The molecules of carbon dioxide, glucose, and water are illustrated below:
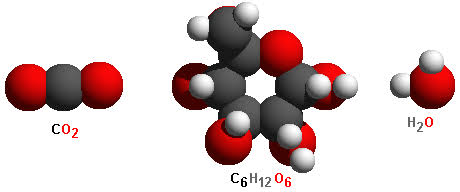
You can see in the above picture that a water molecule is comprised of two hydrogens and one oxygen atom. Similarly, a molecule of carbon dioxide contains one atom of carbon element and two atoms of oxygen element. Thus, atoms of different elements are present in a molecule of a compound in a fixed and definite ratio and this ratio is the characteristics properties of a particular compound.
And it is important to note that the properties of a compound are different from those of the constituents elements. For example, hydrogen and gases are molecules but their combination gives us water which is in a liquid state.
It is interesting to note that the hydrogen gas burns with a pop sound when it is exposed to the flame and the oxygen supports the combustion of the fuel, but water is used as a fire extinguisher. Moreover, the constituents of the compound cannot be separated by using the physical methods into simpler substances. They can be separated by using chemical methods.
Properties of compounds
Some properties of compounds are given below:
- Constituents are present in definite proportion by mass.
- The properties of a compound are different from the properties of its constituents.
- Formation of a compound is generally accompanied by evolution of energy in the form of heat or light.
- It has a fixed melting point and boiling point.
- A compound is always homogeneous in nature.
Stay tuned with Laws Of Nature for more useful and interesting content.